Functional implications of opioids in healthy and diseased brain
Opioid systems in epilepsy and epileptogenesis
With a prevalence of 1-2% worldwide, epilepsy is one of the most frequent neurological diseases affecting people of all ages. Of the 870 million people living in the European Region, over 5 million suffer from epilepsy. Epilepsy has major adverse effects on social and psychological well-being, including social isolation, stigmatization, or disability, thus resulting in lower educational achievement and worse employment outcomes.In line with this, the International League Against Epilepsy (ILAE) defined epilepsyas “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures and by the neurobiologic, cognitive, psychological, and social consequences of this condition.”
Epilepsy comprises a group of chronic neurological diseases characterized by epileptic seizures as a result of excessive electrical discharges in a group of brain cells. Epileptic seizures are episodes varying from brief and nearly undetectable to prolonged convulsions, that may involve a part of the body (partial) or the entire body (generalized), and are sometimes accompanied by loss of consciousness and control of bowel or bladder function. The cause of most cases of epilepsy (ca. 60%) is unknown, although some people develop epilepsy as the result of brain injury, stroke, brain tumor, and substance use disorders. Epilepsies caused by genetic, congenital, or developmental conditions are more common among younger people, while brain tumors and strokes are more likely in older people.
About 70% of all epilepsy patients suffer from focal seizures arising from a distinct brain region, the temporal lobe. Mesial temporal lobe epilepsy (mTLE, with the hippocampus as epileptogenic focus) is considered as one of the most frequent types of epilepsy. One main factor responsible for neuronal losses and seizure induction is excessive glutamate release, which may result from impaired inhibitory signaling. The mainstay treatment of epilepsy relys on antiepileptic drugs (AEDs), mostly for the person's entire life. Notably, 30-50% of patients are refractory to presently available pharmacological treatments. The currentpharmacotherapies of epilepsycause a number of side effects (e.g. sedation, nausea, depression, headache, ataxia) in 10% to 90% of people. In 2008, the FDA issued a black-box warning that several AEDs increase the risk of suicidal thoughts and behavior among users [12]. In those patients whose seizures do not respond to AEDs or neuro-stimulation, surgical resection of the epileptogenic focus remains the ultimate solution. Besides, less than 50% reach seizure freedom for at least one year.
To date, the prevailing means for treatment of patients suffering from epilepsies have been proven to be unable to offer satisfactory long-term solutions, stressing the need (i) for innovative and superior treatments, and (ii) the development of new mechanism-based treatment strategies that specifically modulate sclerosis and altered neurotransmission associated with refractory epilepsies. Therefore, research efforts are needed towards overcoming the limitations of present therapies, with the final goal to improve treatment efficacy, patient compliance and to reduce complications.
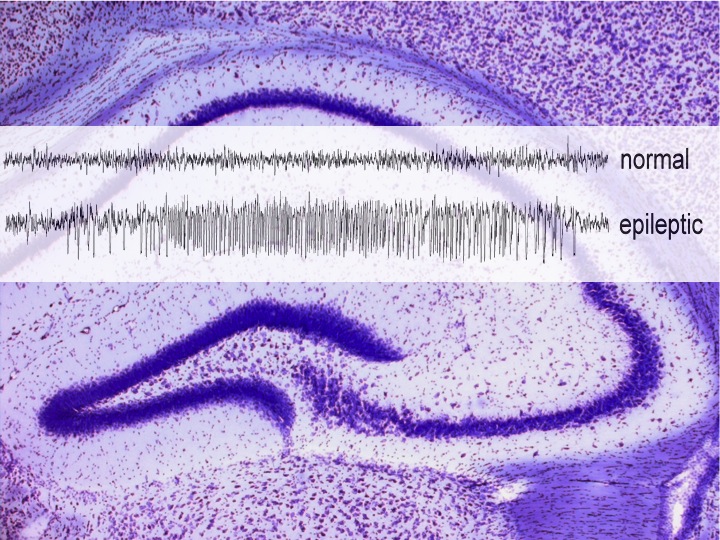
Since the early 1980s, there has been accumulated evidence that Dyn act as modulators of neuronal excitability in vitro. The potentiation of endogenous anti-ictal mechanisms by opioid drugs and Dyn was shown in animal models of epilepsy. In line with these findings, the deletion of pDynin mice and low Dyn expression in human brain is associated with increased epilepsy vulnerability. In contrast to other neuropeptides (i.e. neurokinin B, neuropeptide Y), which are expressed in increased levels and even found in novel brain areas in animal models of temporal lobe epilepsy (TLE), pDyn expression appears reduced after an initial increase in most epilepsy models. By contrast, KOR expression seems unchanged under epileptic conditions, and KOR stimulation can suppress seizures. We previously demonstrated that endogenous Dyn as well as synthetic agonists acting on the KOR mediates anticonvulsant, antiepileptogenic and neuroprotective effects. However, dysphoric and hallucinogenic side effects limit the clinic applicability of currently available KOR agonists, which became evident during early clinic trials of spiradoline and enadoline as analgesics or aquaretics. Using 6’-GNTI, we now provided the proof of principle, that anticonvulsant and aversive effects may be separated, toward enhanced therapeutic benefits of epilepsy patients. Further development of such drug candidates is our primary goal.
Role of opioid systems in emotional control.
Mood disorders are one of the most common disorders in developed countries. The overall prevalence of anxiety disorders was estimated to be about 2 % to 10 %, potentially reaching 20 % in the elderly. Moreover, stress conditions affect broad numbers of persons, potentially resulting in depression or substance abuse. We and others previously demonstrated that endogenous dynorphin acting on kappa opioid receptors exerts anxiogenic effects and is involved in stress response and fear extinction. Moreover, there appears to be a link between sex hormones and the dynorphinergic sytem.
While a lot of emphasis was put in understanding intracellular basis of aversive effects and functional neuroanatomy of kappa opioid receptors, fairly little is known about the functional neuroanatomy of dynorphins. One of our primary aims is to understand which dynorphinergic neurons are involved in the regulation of fear, anxiety and mood to generate a map of the dynorphin / kappa opioid receptor network. Detailed knowledge on the underlying neuroanatomy will help to understand basic mechanisms of pathophysiology and are essential to define novel treatment strategies for. Moreover, understanding the role dynorphin / kappa opioid system in adverse effects helps us to judge the potentials of kappa opioid receptors as drug targets for epilepsy.
Selected Publications (last 5 years)
- Loacker S, Sayyah M, Wittmann W, Herzog H & Schwarzer C (2007) Endogenous dynorphin in epileptogenesis and epilepsy: Anticonvulsant net effect via kappa opioid receptors. Brain, 130, 1017-1028.
- Illig R, Fritsch H & Schwarzer C (2009) Breaking the seals - efficient mRNA detection from human archival paraffin embedded tissue. RNA, 15, 1588-1596
- Schwarzer C (2009) 30 years of dynorphins - new insights on their functions in neuropsychiatric diseases. Pharmacology and Therapeutics, 123, 353-370.
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H & Schwarzer C (2009) Prodynorphin derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology, 34, 775-785.
- Khom S, Strommer B, Ramharter J, Schwarz T, Schwarzer C, Erker T, Ecker GF, Mulzer J & Hering S (2010) Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands - in vitro and in vivo characterization. British Journal of Pharmacology, 161, 65-78.
- Schunk E, Aigner C, Stefanova N, Wenning G, Herzog H & Schwarzer C (2011) Kappa opioid receptor activation blocks progressive neurodegeneration after kainic acid injection. Hippocampus, 21, 1010-1020.
- Kastenberger I, Lutsch C & Schwarzer C (2012) Activation of the G-protein-coupled receptor GPR30 induces anxiogenic effects in mice, similar to oestradiol. Psychopharmacology, 221, 527-535.
- Kastenberger I, Lutsch C, Herzog H & Schwarzer C (2012) Influence of sex and genetic background on anxiety-related and stress-induced behaviour of prodynorphin-deficient mice.
- Ménard C, Tse Y-C; Cavanagh C, Chabot j-G, Herzog H, Schwarzer C, Wong T-P & Quirion R (2013) Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety and rescues loss of group 1 metabotropic glutamate receptor function in aging. The Journal of Neuroscience, 31, 12792-804
- Romero-Picó A, Vázquez MJ, González-ToucedaD, FolgueiraC, Skibicka KP, Alvarez-Crespo M, Van Gestel MA, Velásquez DA, Schwarzer C, Herzog H, López M, Adan RA, Dickson SL,Diéguez C & Nogueiras R (2013) Hypothalamic kappa opioid receptor modulates the orexigenic effect of ghrelin. Neuropsychopharmacology, 38, 1296-1307
- Kardon A, Polgár E, Hachisuka J, Snyder L, Cameron D, Savage S, Cai X, Kamup S, Fan CR, Hemenway G, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Futura T, Kaneko T, Koerber HR, Todd AJ & Ross S (2014) Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron, 82, 573-86
- Burtscher J, Zangrandi L, Schwarzer C* & Gnaiger E* (2015) Differences in mitochondrial function in homogenated samples from healthy and epileptic specific brain tissues revealed by high-resolution respirometry. Mitochondrion, 25:104-112
- Zangrandi L, Burtscher J, MacKay J, Colmers W & Schwarzer C (2016) The G-protein biased partial kappa opioid receptor agonist 6’-GNTI blocks hippocampal paroxysmal discharges without inducing aversion, British Journal of Pharmacology, in press DOI: 10.1111/bph.13475
International collaborations
- Prof. R. Heilbronn, Department of Virology, Campus Benjamin Franklin, Charité – Medical School, Berlin
- Prof. H. Herzog, Garvan Institute of Medical Research, Sydney, Australia
- Prof. Dr. M. Holtkamp, Department of Neurology, Epilepsy-Center Berlin-Brandenburg, Charité-Universitätsmedizin Berlin, Berlin, Germany.
- Dr. J. Liu, Department of Proteomics and Signal Transduction, Max-Planck Institute of Biochemistry, Martinsried, Germany.
Forschung
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.





