Functional implications of opioids in healthy and diseased brain
Opioid systems in epilepsy and epileptogenesis
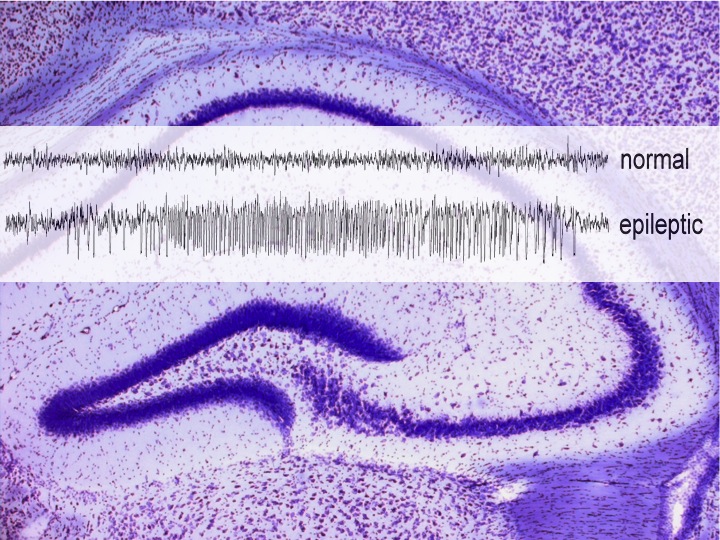
Epilepsy is one of the most frequent neurological diseases, which presently cannot be cured. A high number of patients are refractory to pharmacological treatment, rendering surgical removal of parts of the brain as ultimate solution. Therefore, there is tremendous need to develop novel treatment options. Potentially even more important is the development of antiepileptogenic drugs, which prohibit the generation of the disease and thereby prevent neuronal damage, functional brain deficits and life-long treatment.
We investigate the role of the endogenous dynorphin / kappa opioid receptor system in epilepsy and antiepileptic properties of kappa opioid receptor agonists. The anticonvulsant actions of kappa opioid receptor agonist are fairly well understood. However, we are interested in the effects of such drugs in pharmaco-resistant epilepsy. Moreover, we want to gain insight into potential side-effects of kappa opioid receptor agonist treatment through investigation of behavioral and neurochemical alterations in response to treatment with biased and anbiased agonists. This is an important aspect considering the fact, that antiepileptic drugs are applied for very long periods.
A special interest is the developmental process of epilepsy, termed epileptogenesis. Although a number of animal models resemble several aspects of epileptogenesis, this process is not well understood. In fact, the lack of understanding the neurobiochemical background of epileptogenesis did not allow the introduction of antiepileptogenic drugs in clinics so far. Our aim is the identification of biomarkers for the progression of epileptogenesis by combination of in-vivo EEG recordings, neuropathological, neurobiochemical and functional investigations. On the other hand, we presently investigate the potentials of kappa opioid agonists as antiepileptogenic drugs.
In recent years, we provided evidence, that the activation of kappa opioid receptors plays an important role in epileptogenesis. Thus, dynorphin (the main endogenous agonist of kappa opioid receptors) deficient mice display faster progression and more neurodegeneration in models of epileptogenesis than wild-type animals. This is in line with the observation, that a mutation in the human prodynorphin promoter, resulting in reduced expression of dynorphin, is associated with an increased risk to develop temporal lobe epilepsy. Based on this, we presently investigate the influence of kappa opioid receptor agonist treatment on the progression of epileptogenesis.
Role of opioid systems in emotional control.
Mood disorders are one of the most common disorders in developed countries. The overall prevalence of anxiety disorders was estimated to be about 2 % to 10 %, potentially reaching 20 % in the elderly. While gender differences in severe mental health disorders, such as schizophrenia or bipolar disorders, are hardly noticeable, women score markedly higher in the prevalence of depression and anxiety disorders. Unlike physical symptoms, internalizing mental disorders were still significantly more frequent in women after correction for the socio-economic gradient. This difference manifests after puberty, precipitating the importance of sexual hormones. Changes in mood throughout the estrous cycle display strong inter-individual differences, with a subgroup of women experiencing severe premenstrual anxiety symptoms.
We recently demonstrated that endogenous dynorphin acting on kappa opioid receptors exerts anxiogenic effects in male mice. Anxiogenic properties of dynorphin were also observed by other groups, which were accompanied also with dysphoric effects. In a recent study we investigate the influence of endogenous dynorphin on anxiety-like behaviour during single stages of the estrous cycle in female mice. This study revealed marked alterations in trait anxiety along the estrous cycle in wild-type, but not dyn deficient mice. Moreover, we were able to identify the membrane bound G-protein coupled estrogen receptor GPER1 as potential candidate causing aversive effects in hormone replacement therapy. Our data suggest an interaction of the estrogen and dynorphin systems. In fact such an interaction was recently shown in cycle dependent analgesic effects.
Selected Publications (last 5 years)
- Loacker S, Sayyah M, Wittmann W, Herzog H & Schwarzer C (2007) Endogenous dynorphin in epileptogenesis and epilepsy: Anticonvulsant net effect via kappa opioid receptors. Brain, 130, 1017-1028.
- Illig R, Fritsch H & Schwarzer C (2009) Breaking the seals - efficient mRNA detection from human archival paraffin embedded tissue. RNA, 15, 1588-1596
- Schwarzer C (2009) 30 years of dynorphins - new insights on their functions in neuropsychiatric diseases. Pharmacology and Therapeutics, 123, 353-370.
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H & Schwarzer C (2009) Prodynorphin derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology, 34, 775-785.
- Khom S, Strommer B, Ramharter J, Schwarz T, Schwarzer C, Erker T, Ecker GF, Mulzer J & Hering S (2010) Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands - in vitro and in vivo characterization. British Journal of Pharmacology, 161, 65-78.
- Schunk E, Aigner C, Stefanova N, Wenning G, Herzog H & Schwarzer C (2011) Kappa opioid receptor activation blocks progressive neurodegeneration after kainic acid injection. Hippocampus, 21, 1010-1020.
- Kastenberger I, Lutsch C & Schwarzer C (2012) Activation of the G-protein-coupled receptor GPR30 induces anxiogenic effects in mice, similar to oestradiol. Psychopharmacology, 221, 527-535.
- Kastenberger I, Lutsch C, Herzog H & Schwarzer C (2012) Influence of sex and genetic background on anxiety-related and stress-induced behaviour of prodynorphin-deficient mice.
International collaborations
- Prof. R. Heilbronn, Department of Virology, Campus Benjamin Franklin, Charité – Medical School, Berlin
- Prof. H. Herzog, Garvan Institute of Medical Research, Sydney, Australia
- R. Nogueiras, PhD, Department of Physiology, Faculty of Medicine, University of Santiago de Compostela, Spain.
- DDr. Christiane Otto, TRG Women´s Healthcare, Bayer Schering Pharma AG, Berlin
- Prof. R. Quirion, Douglas Mental Health University Institute, Department of Psychiatry, McGill University, Montréal, Canada.
Research
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.






