Interaction of fear and hunger
Role of neuropeptides
Emotions control evolutionarily conserved and goal-directed behavior that is central to survival in a natural environment. Imbalance within emotional circuitries may result in malfunctioning and manifestation of affective disorders. Although neurobiological substrates of emotionally controlled circuitries are increasingly evident, their mutual influences and the underlying mechanisms are not. We are investigating the interaction between emotionally controlled survival circuits, in particular those for fear and hunger. We specifically focus on the role of evolutionary conserved neuropeptides and their involvement in modulating brain activities that are central to fear, fear extinction and energy homeostasis (Verma et al., 2016b).
In our research projects we want to study
- Interaction of hunger and fear with a specific focus on neuropeptide systems
- Role of Neuropeptide Y (NPY) and in particular Y2 receptors in fear and fear extinction
- Impact of adult neurogenesis on specific aspects of fear processing focusing on the role of neuropeptides
- Characterization of the Neurokinin B (NKB) system in limbic brain areas and its relation to survival circuits
To meet this challenge we employ highly-specific genetic manipulation techniques, where specific genes are deleted or re-introduced into a knock-out background only in restricted cell types and localized brain areas. We also manipulate these neurons permanently by tetanus toxin or caspase 3 or temporarily on different time scales by optogenetic and chemogenetic methods. Combining viral vectors with tetracycline-controlled transcriptional regulation in transgenic animals allows us to re-activate specific memory traces or emotionally relevant neuronal ensembles during different behavioral challenges. Our aim is to identify the neuronal engrams and the underlying molecular machinery that is necessary and sufficient to drive survival circuitries.
Current research projects
Role of neurokinin B in conditioned fear (MUI-Start ST2013042001 & FWF Project P 29952)
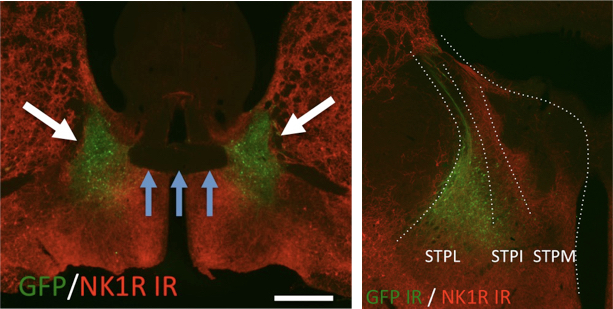
The aim of this study is to investigate the role of the tachykinin neurokinin B (NKB) in the modulation of learned fear. NKB is substantially expressed throughout the amygdala complex, a center for emotional processing. By combining transgenic mouse lines (TAC2-Cre) with chemo-genetic manipulations (DREADD-technology) we have investigated the function of NKB neurons in different amygdala nuclei, including also the extended amygdala and the bed nucleus of the stria terminals in a subdivision-specific manner. To comprehensively illustrate these neurons, we reconstructed viral-vector labeled NKB neurons of the BNST and their axonal branching pattern throughout the mammalian brain. The project also includes the visualization and sub-population-specific localization of NKB synapses in selected target neurons. The overall aim is to characterize specifically NKB-expressing neurons of the extended amygdala and to elucidate their relevance for fear processing.
Role of neuropeptide Y in adult neurogenesis and hippocampus-dependent fear learning (FWF Project P 25851)
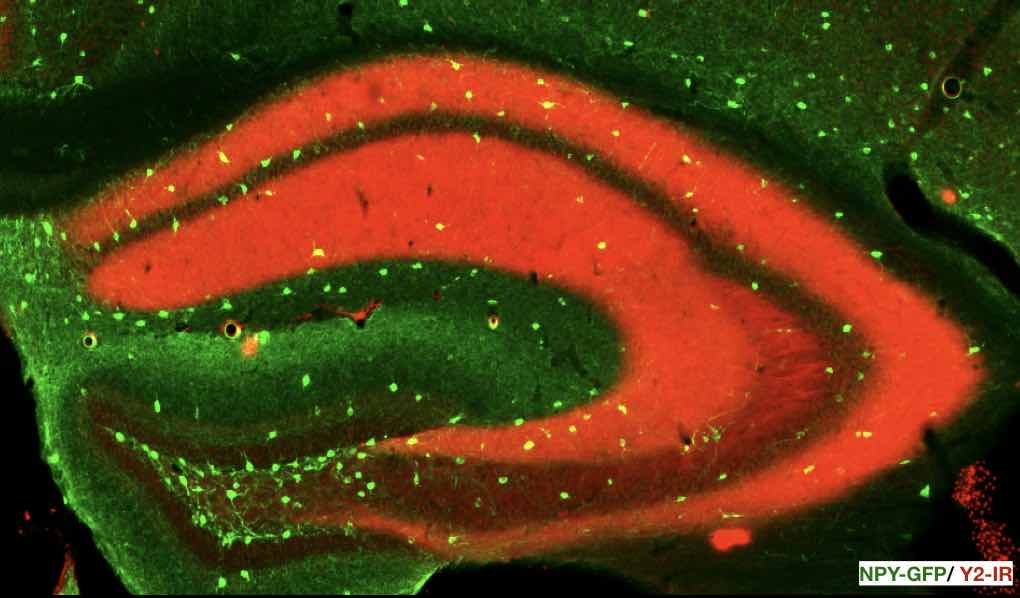
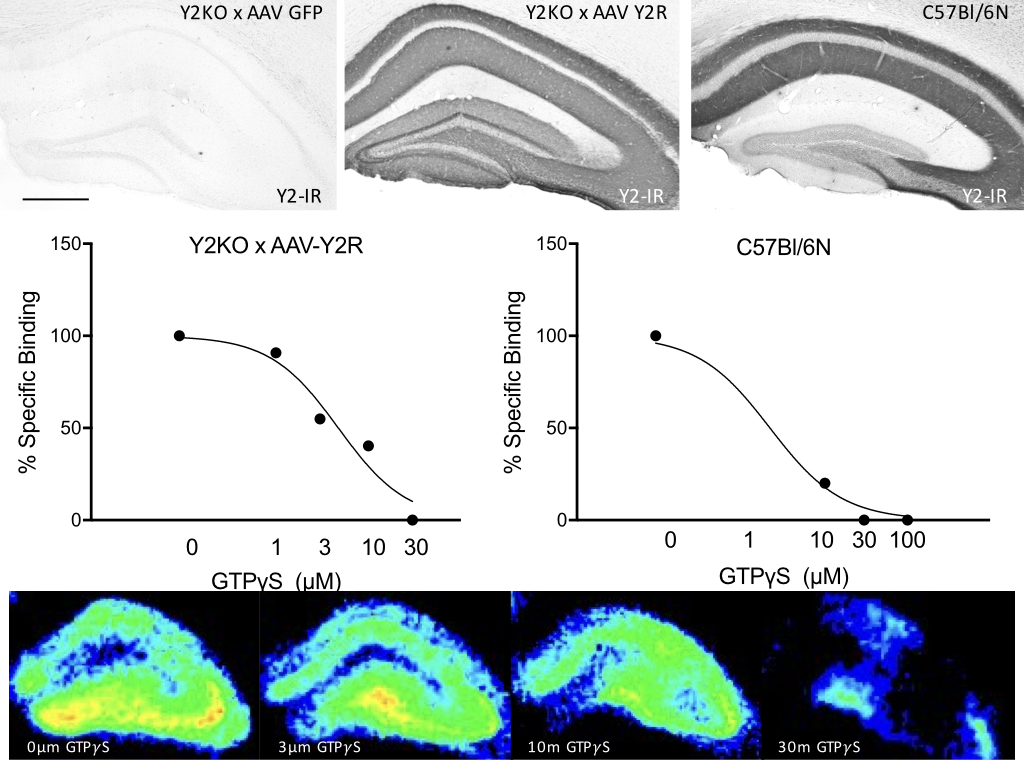
New neurons are constantly generated in the adult mammalian brain. Mounting evidence suggests that adult neurogenesis in the hippocampal dentate gyrus modulates fear learning. We previously demonstrated that the absence of NPY Y2 receptors, a central part of a highly conserved peptidergic neurotransmitter system, results in generalization of cued fear (Verma et al., 2012), and more importantly, also in an inability to differentiate between similar fear contexts. This is of considerable relevance, since fear generalization is a hallmark of post traumatic stress disorders (PTSD). In fact, fear context discrimination learning was frequently associated with the number of newly born neurons in the adult hippocampal dentate gyrus. In this research project we are investigating the role of hippocampal Y2 receptors in emotional and non-emotional learning and we are focusing on possible interactions with adult neurogenesis. We combine different transgenic mouse lines with viral vectors for the exact birth-dating of newly born neurons and to specially modulate these neurons during emotional and memory-acquisition challenges.
Past research projects
Role of neuropeptides in fear and anxiety (FWF Project P 22830)
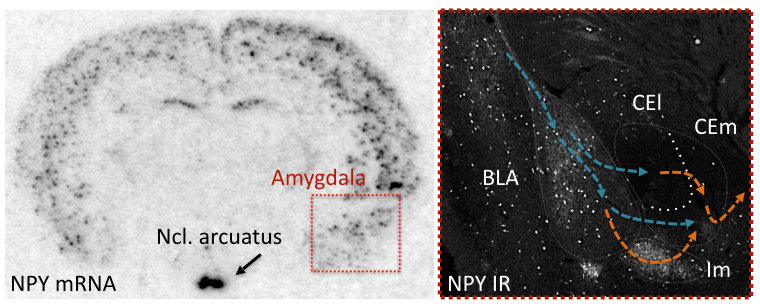
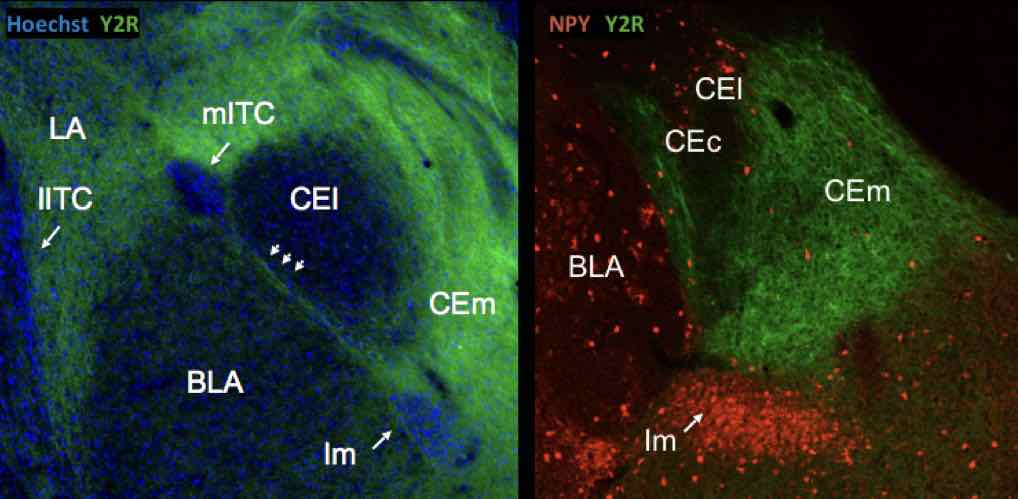
In the frame of this project we were investigating the role of the anxiolytic neuropeptide transmitter Neuropeptide Y (NPY) in models of learned fear and fear extinction. Our main finding was that NPY suppresses fear expression and promotes fear extinction by Y1 and Y2 receptors (Verma et al., 2012). A fear suppressing effect of Y2 receptor activation was localized to the central nucleus of the amygdala (Verma et al., 2015). Here, NPY-containing neurons were projecting towards the bed nucleus of the stria terminalis (BNST) and vice versa, NPY-containing neurons of the BNST were targeting the CEA (Wood et al., 2015).
Since NPY is not only an anxiolytic and fear-suppressing neuromodulator, but also essentially involved in food intake, we interrogated a putative relation between these two life-sustaining circuitries. We demonstrated that hunger suppresses the consolidation of fear and facilitates the extinction of an acquired fear memory (Verma et al., 2016b). Thus, activation of the hunger-circuit may have considerable consequences for the therapy of human anxiety disorders, which are characterized by dys-regulated fear. Along this line, we also showed that for instance pancreatic polypeptide, another member of the NPY-family, was facilitating fear extinction in a state of hunger, but was without effect in saturated individuals (Verma et al., 2016a). Genetic deletion of the designated receptor, the Y4 receptor, on the other hand resulted in impaired fear extinction. Thus, the circuitry of food-intake and processing of emotional aversive stimuli, such as fear or fear extinction, may have by far more interaction-nodes that initially thought.
A main focus of ongoing follow-up research is the investigation of the underlying circuitries by ex vivo electrophysiology and in vivo modulatory influences of neuronal subtypes and transmitter systems as well as the activation of synthetic memory traces.
Dopaminergic and neuropeptide Y modulation of fear extinction
We have previously confirmed that a facilitation of feed-forward inhibition from the basolateral amygdala to the centromedial amygdala was associated with successful fear extinction (Verma et al., 2016b). This feed-forward inhibition was mediated by medial intercalated neurons that also express high amounts of NPY and dopamine D1 receptors (Wood et al., 2015). Thus, in this project we wanted to elucidate neuromodulators that are interfering with a specific amygdala microcircuit and thus may ultimately be useful for promoting the extinction of pathological fear. The approach combined ex vivo electrophysiology in amygdala slices with optogenetics and behavioral testing and specially focused on the interaction between monoaminergic and peptidergic neurotransmitters.
Selected publications
Hörtnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G (2013) Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 236:345–372.
Jagirdar R, Drexel M, Bukovac A, Tasan RO, Sperk G (2015a) Expression of class II HDACs in two mouse models of temporal lobe epilepsy. J Neurochem
Jagirdar R, Drexel M, Kirchmair E, Tasan RO, Sperk G (2015b) Rapid changes in expression of class I and IV histone deacetylases during epileptogenesis in mouse models of temporal lobe epilepsy. Exp Neurol 273:92–104.
Nguyen NK, Sartori SB, Herzog H, Tasan R, Sperk G, Singewald N (2009) Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse 63:236–246.
Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, Holzer P (2008) Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav 7:532–542.
Schmid E, Nogalo M, Bechrakis NE, Fischer-Colbrie R, Tasan R, Sperk G, Theurl M, Beer AG, Kirchmair R, Herzog H, Troger J (2012) Secretoneurin, substance P and neuropeptide Y in the oxygen-induced retinopathy in C57Bl/6N mice. Peptides 37:252–257.
Sperk G, Wieselthaler-Holzl A, Pirker S, Tasan R, Strasser SS, Drexel M, Pifl C, Marschalek J, Ortler M, Trinka E, Heitmair-Wietzorrek K, Ciofi P, Feucht M, Baumgartner C, Czech T (2012) Glutamate decarboxylase 67 is expressed in hippocampal mossy fibers of temporal lobe epilepsy patients. Hippocampus 22:590–603.
Tasan RO, Bukovac A, Peterschmitt YN, Sartori SB, Landgraf R, Singewald N, Sperk G (2011) Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience 183:71–80.
Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G (2009) Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience 158:1717–1730.
Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G (2010) The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci 30:6282–6290.
Tasan RO, Verma D, Wood J, Lach G, Hormer B, de Lima TC, Herzog H, Sperk G (2015) The role of Neuropeptide Y in fear conditioning and extinction. Neuropeptides
Verma D, Hormer B, Made V, Beck-Sickinger A, Herzog H, Sperk G, Tasan RO (2016a) Pancreatic polypeptide and its central Y4 receptors are essential for cued fear extinction and permanent suppression of fear. Br J Pharmacol
Verma D, Tasan RO, Herzog H, Sperk G (2012) NPY controls fear conditioning and fear extinction by combined action on Y(1) and Y(2) receptors. Br J Pharmacol 166:1461–1473.
Verma D, Wood J, Lach G, Herzog H, Sperk G, Tasan R (2016b) Hunger Promotes Fear Extinction by Activation of an Amygdala Microcircuit. Neuropsychopharmacology 41:431–439.
Verma D, Wood J, Lach G, Mietzsch M, Weger S, Heilbronn R, Herzog H, Bonaventure P, Sperk G, Tasan RO (2015) NPY Y2 receptors in the central amygdala reduce cued but not contextual fear. Neuropharmacology 99:665–674.
Wood J, Verma D, Lach G, Bonaventure P, Herzog H, Sperk G, Tasan RO (2015) Structure and function of the amygdaloid NPY system: NPY Y2 receptors regulate excitatory and inhibitory synaptic transmission in the centromedial amygdala. Brain Struct Funct
Zambello E, Zanetti L, Hedou GF, Angelici O, Arban R, Tasan RO, Sperk G, Caberlotto L (2011) Neuropeptide Y-Y2 receptor knockout mice: influence of genetic background on anxiety-related behaviors. Neuroscience 176:420–430.
Research
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.
NPY and Y2 receptors in the amygdala
NPY and Y2 receptors in the hippocampus
Y2 R re-expression in the hippocampus
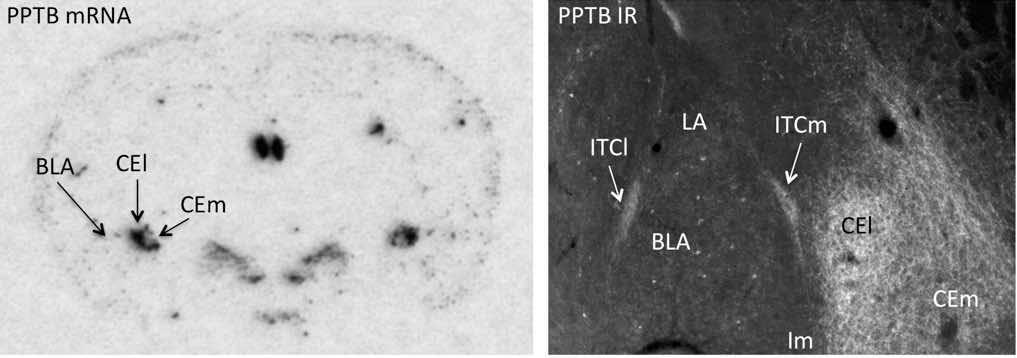
NKB expression in the brain
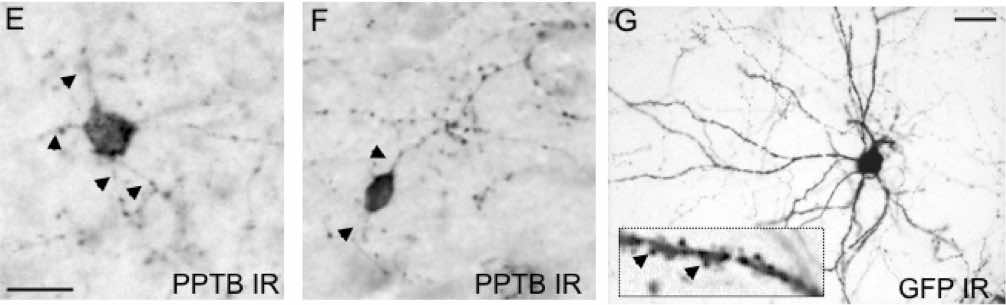
NKB neurons in the basolateral amygdala
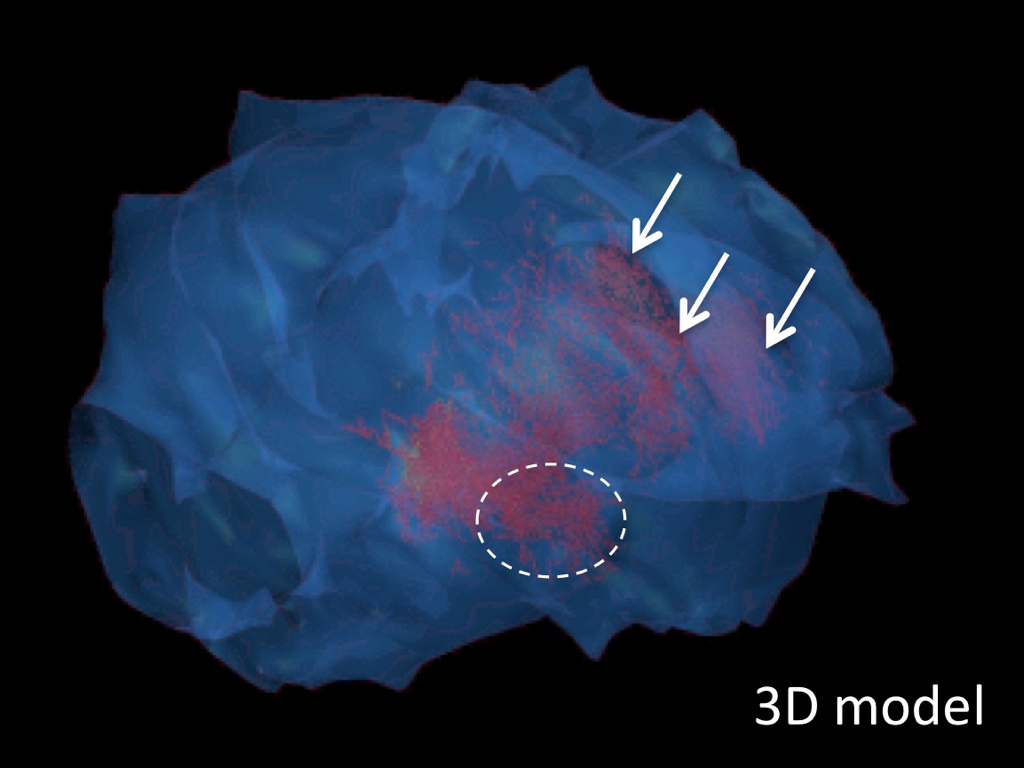
3D model of NKB neurons
NKB in the BNST
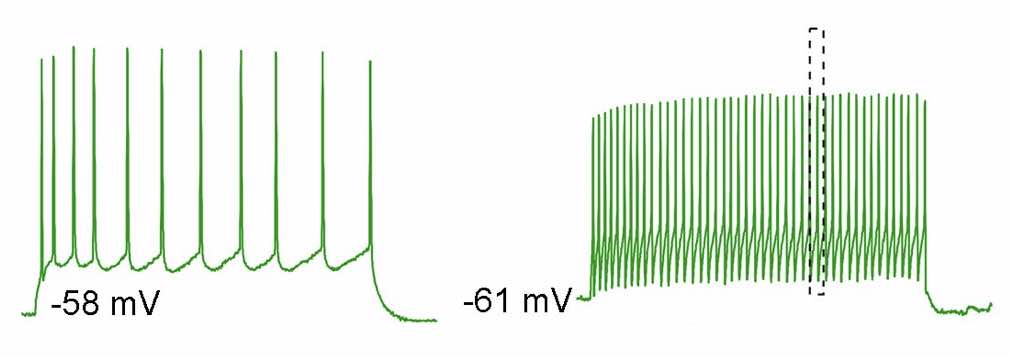
Firing pattern of NKB neurons
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.
NPY and Y2 receptors in the amygdala

NPY and Y2 receptors in the hippocampus

Y2 R re-expression in the hippocampus

NKB expression in the brain

NKB neurons in the basolateral amygdala

3D model of NKB neurons

NKB in the BNST

Firing pattern of NKB neurons






