Peptidergic transmitters
Molecular aspects of hormone and transmitter storage in large dense core vesicles
The neuronal synaptic transmission is mediated by the release of neurotransmitters and neuropeptides from large dense core and synaptic vesicles into the synaptic cleft which then interact with specific receptors on the postsynaptic plasma membrane. The catecholamine storage vesicles of the adrenal medulla, the so-called chromaffin granules, represent an ideal working model for one of the two types of these organelles, the large dense core vesicles (LDV).
We have characterized proteins and peptides present in the content as well as in the surrounding membrane of chromaffin granules in great detail. Briefly, our main findings included:
i) the identification of several glycoproteins of the LDV membrane, i.e. GPII (Fischer-Colbrie et al., 1982; Obendorf et al., 1988), GPIII (Fischer-Colbrie et al., 1982, 1984; Laslop et al., 1993, now also named clusterin or ApoJ), GPIV (Fischer-Colbrie et al., 1982; Margolis et al., 1988; Getlawi et al., 1996) and the carboxypeptidase H (Laslop et al., 1986). GPIII recently gained interest as a novel chaperon secreted from many cells and GPIV was identified as a subunit of the v-ATPase.
ii) the detailed characterization of the main secretory proteins of adrenergic LDV, the so-called chromogranins, comprising chromogranins A and B and secretogranin II. We have demonstrated that chromogranin A is not only confined to the LDV of the adrenal medulla but expressed in many neuronal and endocrine cells (Cohn et al., 1982). Thus, chromogranin A is considered today as standard marker for neuroendocrine tissues and tumours derived from them. A new isolation procedure led to the identification of chromogranin B (Fischer-Colbrie et al., 1985), a protein similar to chromogranin A in certain aspects. For secretogranin II, the function as the precursor of the novel neuropeptide secretoneurin was established (Kirchmair et al., 1993, see below). Recently, a novel LDV protein named NESP55, was cloned by us (Ischia et al., 1997, see below), which is genomically imprinted and expressed only from the maternal allele.
iii) The biosynthesis regulation of LDV proteins by first and second messengers has been studied in vivo and in cell culture. Various in vivo situations and especially pathological conditions resulted in an altered synthesis regulation of chromogranins and neuropeptides yielding a different composition of the secretory content at different times (Sietzen et al., 1987; Fischer-Colbrie et al., 1988; Bauer et al., 1993; Laslop et al., 1994). This remarkable plasticity of the LDV content is now thought to significantly modulate neuronal synaptic transmission.
Current research projects
Secretoneurin, a link between nerves, blood vessels and the immune system
Secretogranin II (Fischer-Colbrie et al., 1995; Fischer-Colbrie et al., 2005) was initially described as a sulfated protein expressed in the pituitary by Zanini and collaborators (Milano, Italy) in 1983. We subsequently demonstrated its occurrence in the chromaffin granules (Fischer-Colbrie et al., 1986) and cloned the cDNA corresponding to bovine secretogranin II (Fischer-Colbrie et al., 1990). In 1993, a peptide generated from secretogranin II by endoproteolytic processing in the brain was discovered by us (Kirchmair et al., 1993).
Subsequent studies revealed that this peptide, named secretoneurin (SN), represented a novel neuropeptide. In the brain, SN is expressed in phylogenetically older parts, overlapping partly, but not completely with established neurotransmitter and neuropeptide systems (Marksteiner et al., 1993). In the peripheral nervous system, SN is found in sympathetic and sensory fibres (Kirchmair et al., 1994). SN has been detected in several endocrine tissues, including the adrenal medulla, endocrine pancreas, posterior pituitary and the endocrine cells of the gastrointestinal tract. Tumours derived from endocrine tissues, including pheochromocytomas, neuroblastomas, ganglioneuromas, pituitary adenomas, C-cell carcinomas or pancreatic islet carcinomas synthesize significant amounts of SN. Therefore, antisera towards SN represent useful histochemical markers for these tumours (Eder et al., 1998; Prommegger et al., 1998). Furthermore, increased levels of SN have been reported in sera from patients suffering from oat cell carcinomas, pheochromocytomas, gastroenteropancreatic endocrine tumours and advanced prostate carcinomas (Ischia et al., 2000).
SN is excellent conserved during evolution, SN homologues have been identified in reptiles, amphibia, birds and even fish (Leitner et al., 1996; Fischer-Colbrie et al., 2005). The region comprising SN comprises the only part of the precursor protein significantly conserved between mammals and fish, demonstrating that SN represents the functional part of the precuror protein secretogranin II (prosecretoneurin).
Preprosecretoneurin (secretogranin II) mRNA is up-regulated in cultured cells by cell depolarization, cAMP, protein kinase C and nerve growth factor and in vivo in animal models for cortical spreading depression, temporal lobe epilepsy (Mahata et al., 1992), ischemia, and after treatment with antipsychotic drugs (Kroesen et al., 1995). Thus, determination of preprosecretoneurin mRNA is an excellent indicator of activated neurons or neurons affected by psychoactive drugs.
Several biological effects have been established for SN. SN releases dopamine from the rat striatum in vivo and in vitro(Saria et al., 1993; Agneter et al., 1995) and has a potent chemotactic effect towards monocytes, eosinophils and endothelial cells (Reinisch et al., 1993; Schratzberger et al., 1997; Kähler et al. 1999). Very recently a potent angiogenic and vasculogenic activity of SN was identified by Kirchmair and Schratzberger (Kirchmair et al., 2004a,b). These effects suggested that SN is involved in conditions contributing to neurogenic inflammation.
Currently we are characterizing the receptor mediating the effects of SN plus the second messenger systems in detail. The biological effects of SN are blocked by preincubation of cells with Pertussis toxin suggesting an involvement of a Gi-protein coupled receptor (GPCR). After establishment of a physiologically active radioligand, specific binding sites were detected on two human monocytic cell lines (Schneitler et al., 1998). These cell lines are currently used to establish the cDNA encoding the SN receptor by expression cloning and RT-PCR using degenerated primers. Second messenger systems, including cAMP, IP3, Ca2+ and MAP kinase, which are responsible for transduction of the SN mediated signal, are analysed in these cells after treatment with various SN concentrations. Furthermore, microphysiometry with a cytosensor is used to identify novel peptidergic SN homologues acting via the SN receptor.
NESP55, a novel genomically imprinted chromogranin-like secretory protein
We have cloned recently a cDNA encoding a novel chromogranin-like protein and suggested the name NESP55 (neuroendocrine secretory protein with a molecular weight of 55,000) for it (Ischia et al., 1997). NESP55 represents a 241 aa protein with an acidic pI and multiple sites for endoproteolytic processing. At the C-terminal end the octapeptide GAIPIRRH is present, which is generated in significant amounts in some tissues, e.g. the adrenal medulla but not in others, e.g the central nervous system.
NESP55 is expressed in the adrenal medulla, the pituitary and in the central and peripheral nervous system (Ischia et al., 1997; Lovisetti-Scamihorn et al., 1999; Leitner et al., 1999; Bauer et al., 1999a) and in endocrine tumors like pheochromocytomas or GEP tumors (Jacobsen et al., 2003). In contrast to other chromogranins NESP55 is not expressed in carcinoids (Srivastava et al., 2004). In the brain, NESP is mainly expressed in the hypothalamus and the brain stem. The noradrenergic locus coeruleus is especially rich in NESP55 mRNA and protein (Bauer et al., 1999b). The cDNA clones corresponding to NESP55 revealed one unusual feature. The mRNA consists of two open reading frames, one encoding NESP and a second one corresponding to exons 2-13 of the Gsa protein (Ischia et al., 1997). From this putative second reading frame most likely no protein is translated. The NESP55 mRNA is tissue specifically spliced, the splice variants differ in the 3' untranslated mRNA encoding Gsa and do therefore not affect NESP55 protein (Weiss et al., 2000). Studies by J. Peters, Harwell, UK and D. Bonthron, Edinburgh, UK demonstrated that the exon encoding NESP55 is part of the GNAS locus and is located in the genome 50 kb upstream of exon1 of Gsa. These groups showed that NESP55 belongs to a cluster of imprinted genes on mouse chromosome 2 including Gsa and XLas. NESP55 is paternally imprinted and transcribed only from the maternal allele and the first genomically imprinted LDV protein. The mechanism leading to genomic imprinting is yet only partially understood, only 200 out of the 100,000 human genes are genomically imprinted.
Currently, we are investigating the distribution, biosynthesis and posttranslational modification of NESP in more detail. The AtT-20 corticotropic cell line, which expresses high amounts of NESP endogenously, is used as model cell-line for pulse chase studies. The regulation of NESP55 biosynthesis by various first and second messengers is studied in PC12 cells. The subcellular localisation is unravelled by generation of various NESP-GFP expression constructs, which are incorporated into primary neuronal cultures by gene gun experiments. Proteolytic processing of NESP55 in various tissues is analysed by fractionation of tissue extracts followed by RIA. In addition, the endopeptidases (PC1, PC2, PACE 4) involved in this process are investigated. The distribution and subcellular localisation of NESP55 in the central nervous system is studies by immunofluorescense and pre-embedding immune-electronmicroscopy using an affinity purified antibody directed against the C-terminal octapeptide GAIPIRRH.
References:
- Agneter E., Sitte H. H., Stöckl-Hiesleitner S., Fischer-Colbrie R., Winkler H., and Singer E. A. (1995) Sustained dopamine release induced by secretoneurin in the striatum of the rat: a microdialysis study. J. Neurochem. 65, 622-625.
- Bauer J. W., Kirchmair R., Egger C., and Fischer-Colbrie R. (1993) Histamine induces a gene-specific synthesis regulation of secretogranin II but not of chromogranin A and B in chromaffin cells in a calcium-dependent manner. J. Biol. Chem. 268, 1586-1589.
- Bauer R., Weiss C., Marksteiner J., Doblinger A., Fischer-Colbrie R., and Laslop A. (1999a) The new chromogranin-like protein NESP55 is preferentially localized in adrenaline-synthesizing cells of the bovine and rat adrenal medulla.Neurosci. Lett. 263, 13-16.
- Bauer R., Ischia R., Marksteiner J., Kapeller I., and Fischer-Colbrie R. (1999b) Localization of neuroendocrine secretory protein 55 messenger RNA in the rat brain. Neuroscience 91, 685-694.
- Cohn D. V., Morrissey J. J., Shofstall R. E., and Chu L. L. H. (1982) Cosecretion of secretory protein-I and parathormone by dispersed bovine parathyroid cells. Endocrinology 110, 625-630.
- Eder U., Fischer-Colbrie R., Kogner P., Leitner B., Bjellerup P., and Winkler H. (1998) Levels and molecular forms of chromogranins in human childhood neuroblastomas and ganglioneuromas. Neurosci Lett 253, 17-20.
- Fischer-Colbrie R., Kirchmair R., Kähler C. M., Wiedermann C. J. and Saria A. (2005) Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci 6, 373-385.
- Fischer-Colbrie R., Laslop A., and Kirchmair R. (1995) Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog. Neurobiol. 46, 49-70.
- Fischer-Colbrie R., Gutierrez J., Hsu C. M., Iacangelo A., and Eiden L. E. (1990) Sequence analysis, tissue distribution and regulation by cell depolarization, and second messengers of bovine secretogranin II (chromogranin C) mRNA. J. Biol. Chem. 265, 9208-9213.
- Fischer-Colbrie R., Iacangelo A., and Eiden L. E. (1988) Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc. Natl. Acad. Sci. USA 85,3240-3244.
- Fischer-Colbrie R., Hagn C., and Schober M. (1987) Chromogranins A, B and C: widespread constituents of secretory vesicles. Ann. NY Acad. Sci. 493, 120-134.
- Fischer-Colbrie R., Hagn C., Kilpatrick L., and Winkler H. (1986) Chromogranin C: a third component of the acidic proteins in chromaffin granules. J. Neurochem. 47, 318-321.
- Fischer-Colbrie R. and Frischenschlager I. (1985) Immunological characterization of secretory proteins of chromaffin granules: chromogranins A, chromogranins B and enkephalin-containing peptides. J. Neurochem. 44, 1854-1861.
- Fischer-Colbrie R., Zangerle R., Frischenschlager I., Weber A., and Winkler H. (1984) Isolation and immunological characterization of a glycoprotein from adrenal chromaffin granules. J. Neurochem. 42, 1008-1016.
- Fischer-Colbrie R., Schachinger M., Zangerle R., and Winkler H. (1982) Dopamine ß-hydroxylase and other glycoproteins from the soluble content and the membranes of adrenal chromaffin granules: isolation and carbohydrate analysis. J. Neurochem. 38, 725-732.
- Getlawi F., Laslop A., Schägger H., Ludwig J., Haywood J., and Apps D. (1996) Chromaffin granule membrane glycoprotein IV is identical with Ac45, a membrane-integral subunit of the granule's H+-ATPase. Neurosci. Lett. 219,13-16.
- Hoflehner J., Eder U., Laslop A., Seidah N. G., Fischer-Colbrie R., and Winkler H. (1995) Processing of secretogranin II by prohormone convertases: importance of PC1 in generation of secretoneurin. FEBS Lett. 360, 294-298.
- Ischia R., Gasser R. W., Fischer-Colbrie R., Eder U., Pagani A., Cubeddu L. X., Lovisetti-Scamihorn P., Finkenstedt G., Laslop A., and Winkler H. (2000) Levels and molecular properties of secretoneurin-immunoreactivity in the serum and urine of control and neuroendocrine tumor patients. J Clin Endocrinol Metab 85, 355-360.
- Ischia R., Hobisch A., Bauer R., Weiss U., Gasser R. W., Horninger W., Bartsch G., Jr., Fuchs D., Bartsch G., Winkler H., Klocker H., Fischer-Colbrie R., and Culig Z. (2000) Elevated levels of serum secretoneurin in patients with therapy resistant carcinoma of the prostate. J Urol 163, 1161-1164.
- Ischia R., Lovisetti-Scamihorn P., Hogue-Angeletti R., Wolkersdorfer M., Winkler H., and Fischer-Colbrie R. (1997) Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J. Biol. Chem. 272, 11657-11662.
- Jakobsen A.-M., Ahlman H., Kölby L., Abrahmansson J., Fischer-Colbrie R. and Nilsson O. (2003) NESP55 , a novel chromogranin-like peptide, is expressed in endocrine tumors of the pancreas and adrenal medulla but not in ileal carcinoids. Br. J. Cancer 88, 1746-1754.
- Kähler C. M., Kaufmann G., Hogue-Angeletti R., Fischer-Colbrie R., Dunzendorfer S., Reinisch N., and Wiedermann C. J. (1999) A soluble gradient of the neuropeptide secretoneurin promotes the transendothelial migration of monocytes in vitro. Eur J Pharmacol 365, 65-75.
- Kirchmair R., Gander R., Hanley A., Silver M., Ritsch A., Sturm W., Kearney M., Fischer-Colbrie R., Kircher B., Winkler H., Ropper A.H., Losordo D.W., Patsch J.R., Schratzberger P. (2004) The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 109, 777-783.
- Kirchmair R., Egger M., Walter D. H., Eisterer W., Niederwanger A., Woell E., Nagl M., Pedrini M., Murayama T., Frauscher S., Hanley A., Silver M., Brodmann M., Sturm W., Fischer-Colbrie R., Losordo D. W., Patsch J. R. and Schratzberger P. (2004) Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation110, 1121-1127.
- Kirchmair R., Leitner B., Fischer-Colbrie R., Marksteiner J., Hogue-Angeletti R., and Winkler H. (1995) Large variations in the proteolytic formation of a chromogranin A-derived peptide (GE-25) in neuroendocrine tissues.Biochem. J. 310, 331-336.
- Kirchmair R., Marksteiner J., Troger J., Mahata S. K., Mahata M., Donnerer J., Amann R., Fischer-Colbrie R., Winkler H., and Saria A. (1994) Human and rat primary C-fibre afferents store and release secretoneurin, a novel neuropeptide. Eur. J. Neurosci. 6, 861-868.
- Kirchmair R., Hogue-Angeletti R., Gutierrez J., Fischer-Colbrie R., and Winkler H. (1993) Secretoneurin - a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 53, 359-365.
- Kroesen S., Marksteiner J., Mahata S. K., Mahata M., Fischer-Colbrie R., Saria A., Kapeller I., and Winkler H. (1995) Effects of haloperidol, clozapine and citalopram on messenger RNA levels of chromogranins A and B and secretogranin II in various regions of rat brain. Neuroscience 69, 881-891.
- Kroesen S., Marksteiner J., Leitner B., Hogue-Angeletti R., Fischer-Colbrie R., and Winkler H. (1996) Rat brain: Distribution of immunoreactivity of PE-11, a peptide derived from chromogranin B. Eur. J. Neurosci. 8, 2679-2689.
- Laslop A., Fischer-Colbrie R., Hook V., Obendorf D., and Winkler H. (1986) Identification of two glycoproteins of chromaffin granules as the carboxypeptidase H. Neurosci. Lett. 72, 300-304.
- Laslop A., Mahata S. K., Wolkersdorfer M., Mahata M., Srivastava M., Seidah N. G., Fischer-Colbrie R., and Winkler H. (1994) Large dense-core vesicles in rat adrenal after reserpine: levels of mRNAs of soluble and membrane-bound constituents in chromaffin and ganglion cells indicate a biosynthesis of vesicles with higher secretory quanta. J. Neurochem. 62, 2448-2456.
- Laslop A., Steiner H. J., Egger C., Wolkersdorfer M., Kapelari S., Hogue-Angeletti R., Erickson J. D., Fischer-Colbrie R., and Winkler H. (1993) Glycoprotein III (clusterin, sulfated glycoprotein-2) in endocrine, nervous, and other tissues: immunochemical characterization, subcellular localization, and regulation of biosynthesis. J. Neurochem. 61, 1498-1505.
- Laslop A., Weiss C., Savaria D., Eiter C., Tooze S. A., Seidah N. G., and Winkler H. (1998) Proteolytic processing of chromogranin B and secretogranin II by prohormone convertases. J. Neurochem. 70, 374-383.
- Leitner B., Fischer-Colbrie R., Scherzer G., and Winkler H. (1996) Secretogranin II: relative amounts and processing to secretoneurin in various rat tissues. J. Neurochem. 66, 1312-1317.
- Leitner B., Lovisetti-Scamihorn P., Heilmann J., Striessnig J., Blakely R. D., Eiden L. E., and Winkler H. (1999) Subcellular localization of chromogranins, calcium channels, amine carriers, and proteins of the exocytotic machinery in bovine splenic nerve. J. Neurochem. 72, 1110-1116.
- Lovisetti-Scamihorn P., Fischer-Colbrie R., Leitner B., Scherzer G., and Winkler H. (1999) Relative amounts and molecular forms of NESP55 in various bovine tissues. Brain Res 829, 99-106.
- Mahata S. K., Marksteiner J., Sperk G., Mahata M., Gruber B., Fischer-Colbrie R., and Winkler H. (1992) Temporal lobe epilepsy of the rat: differential expression of mRNAs of chromogranin B, secretogranin II, synaptin/synaptophysin and p65 in subfields of the hippocampus. Mol. Brain Res. 16, 1-12.
- Margolis R. U., Fischer-Colbrie R., and Margolis R. K. (1988) Poly(N-acetyllactosaminyl) oligosaccharides of chromaffin granule membrane glycoproteins. J Neurochem 51, 1819-1824.
- Marksteiner J., Kirchmair R., Mahata S. K., Mahata M., Fischer-Colbrie R., Hogue-Angeletti R., Saria A., and Winkler H. (1993) Distribution of secretoneurin, a peptide derived from secretogranin II, in rat brain: an immunocytochemical and radioimmunological study. Neuroscience 54, 923-944.
- Obendorf D., Schwarzenbrunner U., Fischer-Colbrie R., Laslop A., and Winkler H. (1988) Immunological characterization of a membrane glycoprotein of chromaffin granules: its presence in endocrine and exocrine tissues.Neuroscience 25, 343-351.
- Prommegger R., Obrist P., Ensinger C., Schwelberger H. G., Wolf C., Fischer-Colbrie R., Mikuz G., and Bodner E. (1998) Secretoneurin in carcinoids of the appendix - immunohistochemical comparison with chromogranins A, B and secretogranin II. Anticancer Res 18, 3999-4002.
- Reinisch N., Kirchmair R., Kähler C. M., Hogue-Angeletti R., Fischer-Colbrie R., Winkler H., and Wiedermann C. J. (1993) Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett. 334, 41-44.
- Saria A., Troger J., Kirchmair R., Fischer-Colbrie R., Hogue-Angeletti R., and Winkler H. (1993) Secretoneurin releases dopamine from rat striatal slices: a biological effect of a peptide derived from secretogranin II (chromogranin C). Neuroscience 54, 1-4.
- Schneitler C., Kähler C., Wiedermann C. J., Hogue-Angeletti R., and Fischer-Colbrie R. (1998) Specific binding of a 125I-secretoneurin analogue to a human monocytic cell line. J. Neuroimmunol. 86, 87-91.
- Schratzberger P., Reinisch N., Prodinger W. M., Kähler C. M., Sitte B. A., Bellmann R., Fischer-Colbrie R., Winkler H., and Wiedermann C. J. (1997) Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J. Immunol. 158, 3895-3901.
- Sietzen M., Schober M., Fischer-Colbrie R., Scherman D., Sperk G., and Winkler H. (1987) Rat adrenal medulla: levels of chromogranins, enkephalins, dopamine ß-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience 22, 131-139.
- Srivastava A., Padilla O., Fischer-Colbrie R., Tischler A.S., Dayal Y. (2004) Neuroendocrine Secretory Protein-55 (NESP-55) expression discriminates pancreatic endocrine tumors and pheochromocytomas from carcinoids of gastrointestinal or pulmonary origin. Am. J. Surg. Pathol. in press.
- Weiss U., Ischia R., Eder S., Lovisetti-Scamihorn P., Bauer R., and Fischer-Colbrie R. (2000) Neuroendocrine secretory protein 55 (NESP55): Alternative splicing onto transcripts of the GNAS gene and posttranslational processing of a maternally expressed protein. Neuroendocrinology 71, 177-186.
- Winkler H. and Fischer-Colbrie R. (1992) The chromogranins A and B: the first 25 years and future perspectives.Neuroscience 49, 497-528.
Forschung
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.
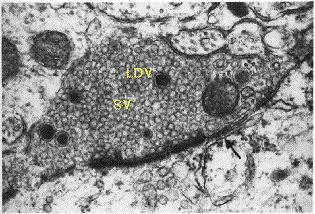
Axon terminal with
large dense core (LDV)
and synaptic vesicles (SV)
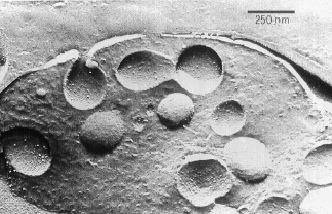
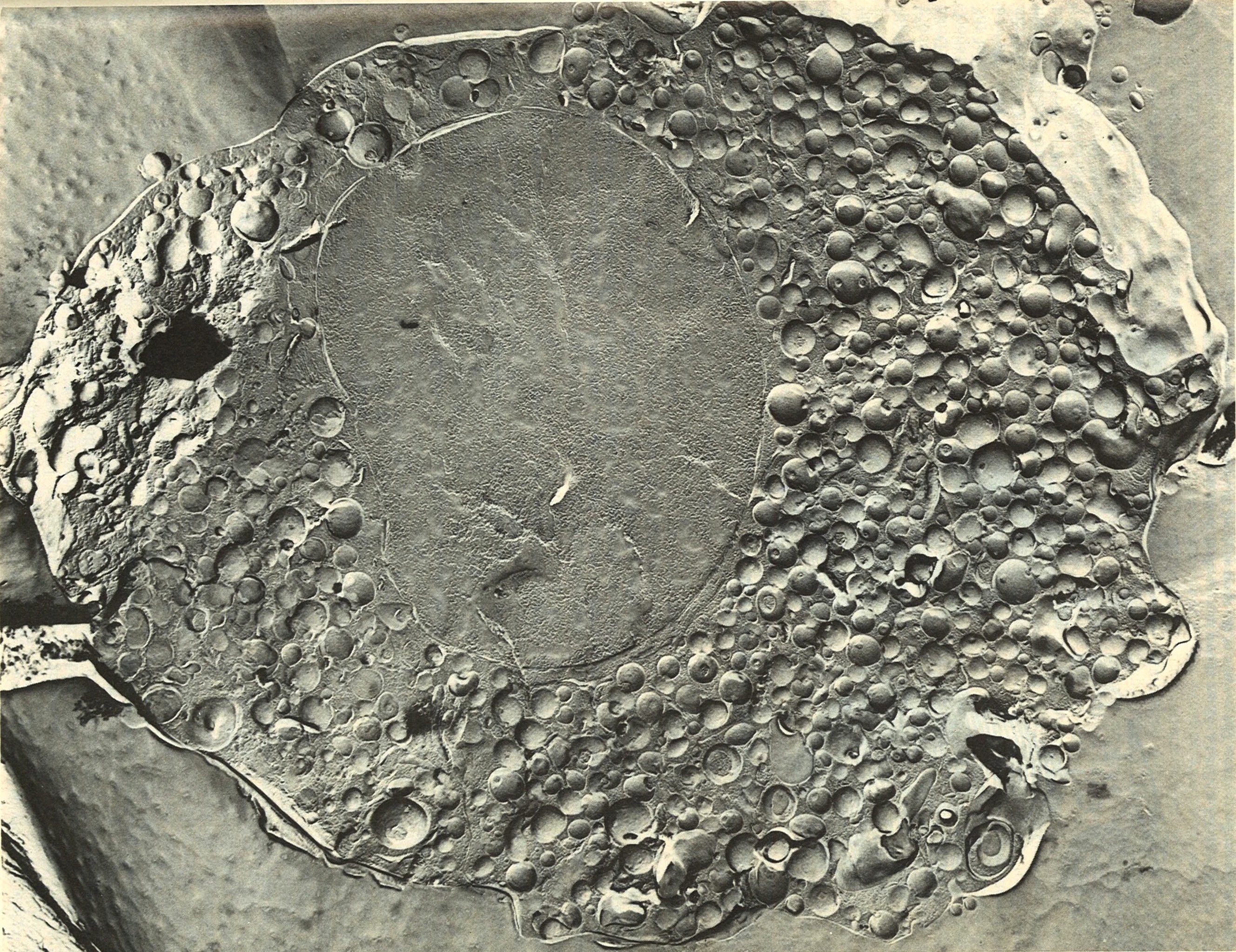
Electronmicroscopic image
of bovine chromaffin granules
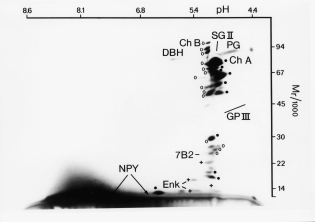
2D gel of secretory proteins
from chromaffin granules
ChA, chromogranin A
SgII, secretogranin II
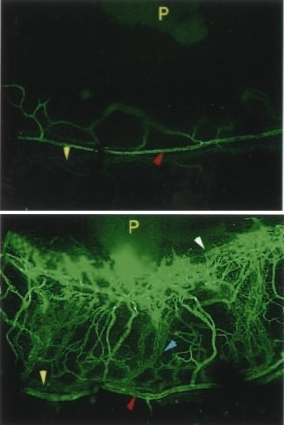
SN-induced angiogenesis
in the limbus vein
(upper panel = control).
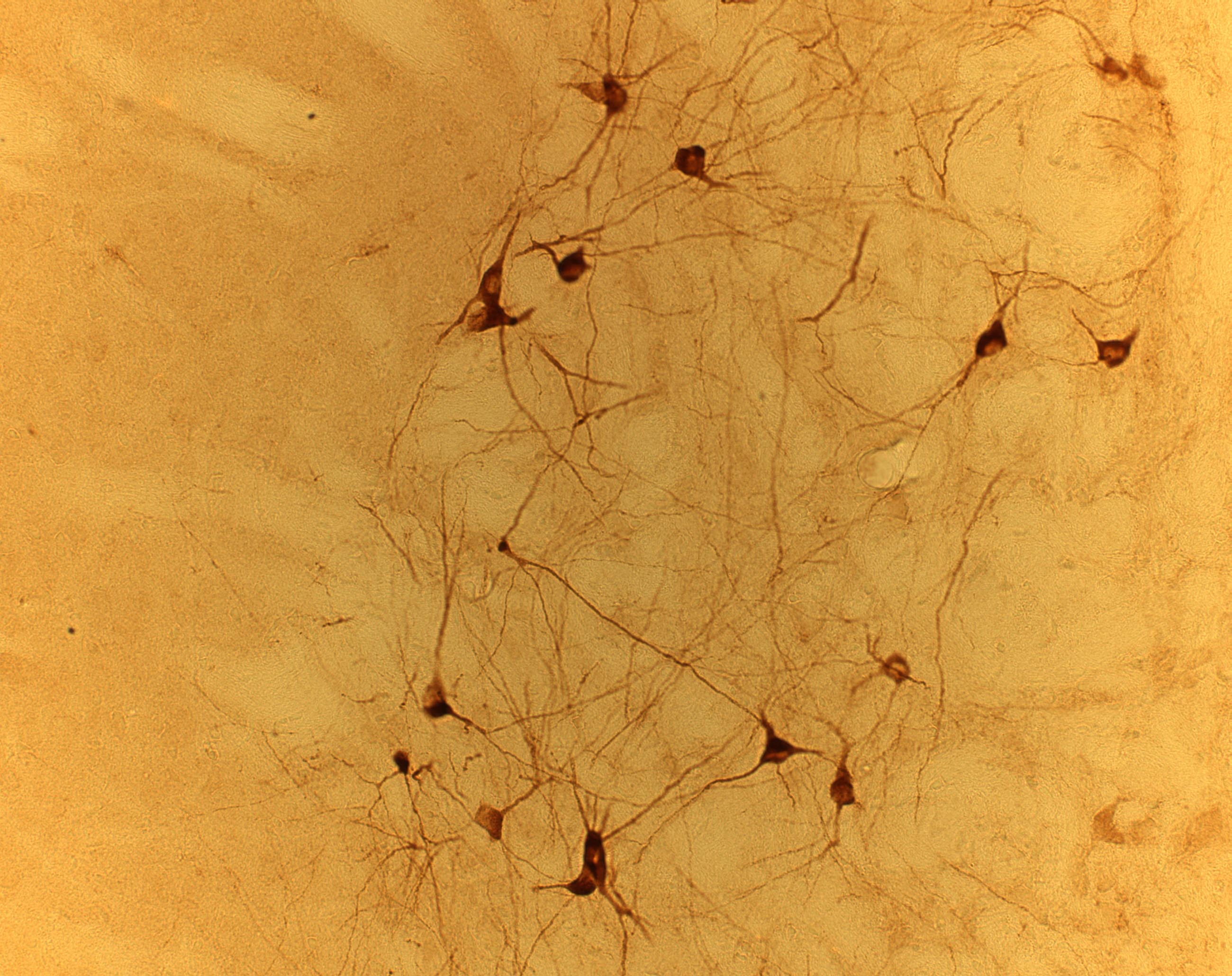
Immunocytochemical analysis
of NESP in the globus pallidus.
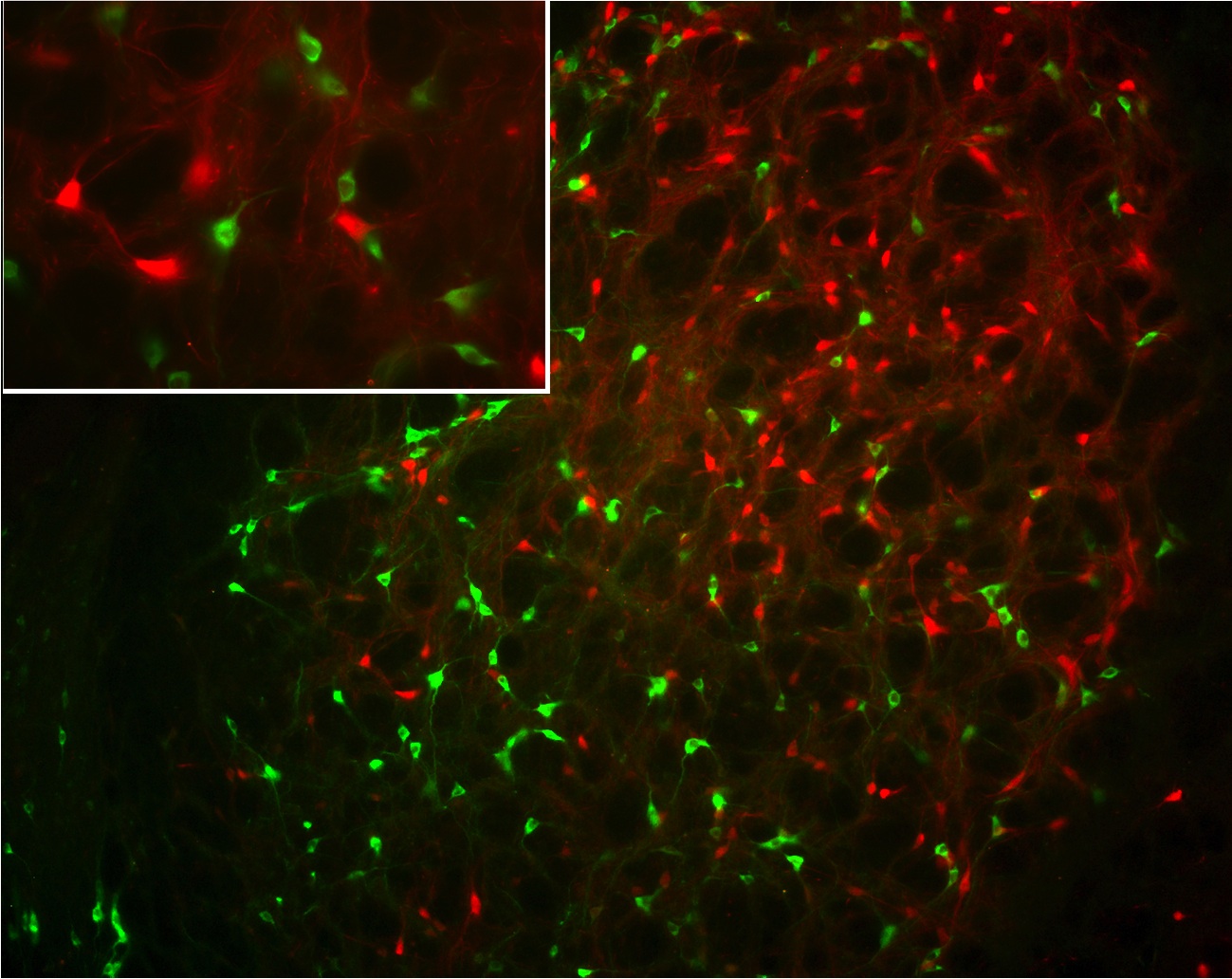
Co-localisation of
NESP55 (green)
and parvalbumin (red)
in the globus pallidus
Kontakt:
Iris Markt
Tel.: +43 (0)512/9003-71201
E-Mail: iris.markt@i-med.ac.at
Fax: +43 (0)512/9003-73200
E-Mail: pharmakologie@i-med.ac.at
Peter-Mayr-Straße 1a
A-6020 Innbruck
Sie finden uns hier.

Axon terminal with
large dense core (LDV)
and synaptic vesicles (SV)

Electronmicroscopic image
of bovine chromaffin granules

2D gel of secretory proteins
from chromaffin granules
ChA, chromogranin A
SgII, secretogranin II

SN-induced angiogenesis
in the limbus vein
(upper panel = control).

Immunocytochemical analysis
of NESP in the globus pallidus.

Co-localisation of
NESP55 (green)
and parvalbumin (red)
in the globus pallidus