Welcome to the Obermair Lab: Neurophysiology of calcium channels
Our research: Voltage-gated calcium channels in normal and diseased nerve cells
In nerve cells the ubiquitous second messenger calcium regulates a variety of vitally important functions including neurotransmitter release, gene regulation, and neuronal plasticity. The entry of calcium into cells is controlled by calcium channels, which open in response to an electrical depolarization of the plasma membrane, the so called voltage-gated calcium channels. These channels consist of a pore forming α1 subunit, which forms the channel allowing the flow of calcium into the cell, as well as the auxiliary β and α2δ subunits.
The latter support the function of the α1 subunit by stabilizing the channel complex and contributing to the localization and expression of the entire channel complex. Because of their wide-spread distribution and their importance for normal brain function, mutations or malfunctions of calcium channels are associated with a variety of neurological diseases. These diseases include migraine, epilepsy, autism, forms of ataxia, chronic pain, mood disorders and recent studies also suggest an involvement in Parkinson’s and Alzheimer’s disease.
Foto: MUI/Presseabteilung; Gerald Obermair mit seinem Team PhD - Student Clemens Schöpf und Stefanie Geisler >> Presseartikel mypoint
The family of α1 subunits consists of 10 genes, seven of which constitute the high-voltage-activated calcium channels of the CaV1 and CaV2 subgroups (α1A to α1F and α1S). With the exception of two specific α1 subunits expressed in skeletal muscle (see research by The Flucher Lab) and in the retina all isoforms are expressed in the nervous system.
Regarding the auxiliary subunits all of the four β subunits and three of the four α2δ subunits are found in the brain. Because all α1, β, and α2δ subunits can form functional channel complexes with each other, this yields the theoretical number of 60 different brain calcium channels (5 α1 X 4 β X 3 α2δ) not considering the existence of multiple splices of each protein! In our research we address the question why nerve cells express so many different calcium channels subunits and whether individual subunits are responsible for specific cellular functions. In this regard we are especially interested in how these channels regulate synapses to allow the communication between nerve cells.
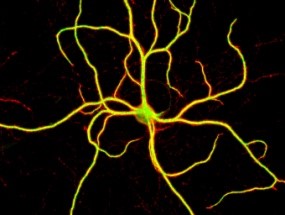
This cellular crosstalk constitutes the basis of all higher brain function such as learning and memory. One hallmark of our research is the focus on the high resolution imaging of calcium channels in nerve cells, which is honoured by the repeated selection of images from our lab for the cover page of scientific journals [Gallery].

Joint research groups of Dr. Obermair (see below) and Dr. Flucher (link)
Our current research efforts focus on two primary topics:
- The role of the CaV1.3 subunit for in the etiology of Parkinson's disease (PD) [Project].
- The neuronal functions of auxiliary α2δ subunits, which are important drug targets for the treatment of epilepsy and chronic pain [Project].
Dr. Ruslan Stanika, Stefanie Geisler, MSc., Assoc.-Prof. Dr. Gerald Obermair, Clemens Schöpf, MSc.
Gerald Obermair
Gerald Obermair,
Division of Physiology
Dept. Physiology and Medical Physics
Medical University of Innsbruck
Schöpfstrasse 41
6020 Innsbruck, Austria.
Phone: +43 512 9003 70841
Gerald.Obermair@i-med.ac.at

Gerald Obermair,
Division of Physiology
Dept. Physiology and Medical Physics
Medical University of Innsbruck
Schöpfstrasse 41
6020 Innsbruck, Austria.
Phone: +43 512 9003 70841
Gerald.Obermair@i-med.ac.at










