Adaptive multi-photon imaging
Multi-photon excitation scanning microscopy such as two-photon excitation fluorescence (TPEF) is one of the most powerful tools for imaging structures deep in biological tissues. It uses infrared excitation wavelengths, which are less prone to scattering compared to visible light and can penetrate significantly farther through seemingly opaque tissues like brain and skin. Also, in TPEF there is no need to sharply image the signal – which is distorted as it propagates through the specimen – onto a camera. Instead, all of the light is collected by a bucket detector and an image is generated by scanning the excitation beam Below is a TPEF image of a microglia cell in mouse brain tissue taken in our lab.
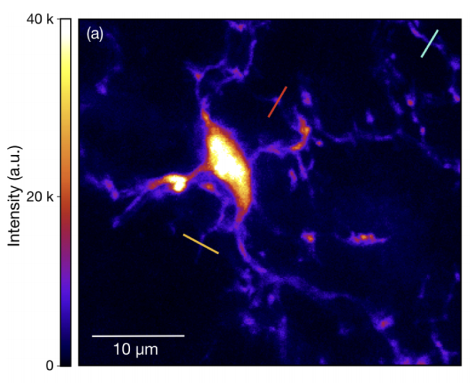
Figure 1 TPEF image of GFP-labelled microglia cell inside fixed mouse brain tissue
However, even TPEF imaging can only be used up to a finite depth. Beyond this, too many excitation photons undergo multiple scattering events, which destroys the imaging focus as shown in Fig. 1.
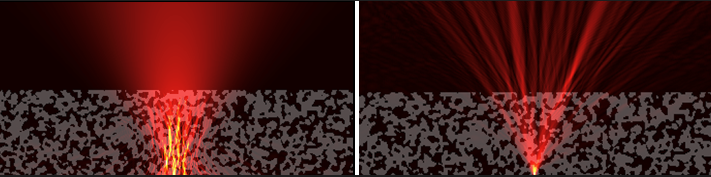
Figure 2 Left: An incident laser beam is scattered by a thick sample, making it difficult to image at great depths; right: Adaptive optics seeks to find and shape optimal wavefronts so that the sample restores the focus rather than destroying it.
In this project, we investigate methods to counteract these scattering events using spatial light modulation and therefore to significantly push the limits of deep tissue imaging. Our focus lies on bringing the technology closer to biological real-world applications. This requires improving on certain key aspects such as correction speed, photon-efficient algorithms and enlarging the corrected field of view.
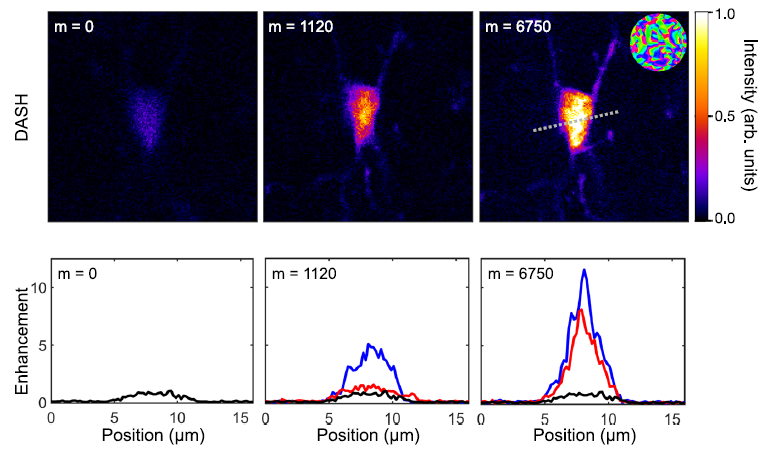 Figure 3 Scattering correction in mouse hippocampal tissue. (Top.) Images of a single microglia cell in mouse hippocampal tissue acquired before correction (m = 0), after the first iteration (m =1120), and after convergence of the DASH algorithm (m = 6750), with the resulting SLM correction mask shown in the inset and scale bar corresponding to 10 μm. Similar results were reproduced five times. (Bottom.) Profiles along the gray, dashed line for no correction (black), DASH correction (blue), and F-SHARP correction (red).
Figure 3 Scattering correction in mouse hippocampal tissue. (Top.) Images of a single microglia cell in mouse hippocampal tissue acquired before correction (m = 0), after the first iteration (m =1120), and after convergence of the DASH algorithm (m = 6750), with the resulting SLM correction mask shown in the inset and scale bar corresponding to 10 μm. Similar results were reproduced five times. (Bottom.) Profiles along the gray, dashed line for no correction (black), DASH correction (blue), and F-SHARP correction (red).Results of this project have been recently highlighted in a contribution to the "Sculptured Light in the Brain Online Series 2021":
Fast converging compensation for deep tissue imaging
Researchers on this project at our institute
- Maximilian Sohmen, PhD (postdoc)
- Juan-David Muñoz-Bolaños, MSc. (PhD student)
- Matthias Dallio, BSc. (Master student)
- Daniel Grünbacher, BSc. (Master student)
- Johannes Locher, BSc. (Master student)
- Prof. Monika Ritsch-Marte
- Prof. Alexander Jesacher (PI)
Funding
“Deep brain vision: 3D adaptive 2-photon microscopy” FWF P32146, 2019 – 2023.
Collaborations
- Michaela Kress, Dept. of Physiology, Medical University of Innsbruck
Publications
[1] Sohmen M., Muñoz-Bolaños J., Rajaeipour P., Monika Ritsch-Marte M., Ataman Ç., and Jesacher A., Optofluidic adaptive optics in multi-photon microscopy, Biomed. Opt. Express 14, 1562-1578 (2023), https://doi.org/10.1364/BOE.481453
[2] Sohmen, M., May, M. A., Barré, N., Ritsch-Marte, M., and A.Jesacher: Sensorless wavefront correction in two-photon microscopy across different turbidity scales. Frontiers in Physics, 10: 884053 (2022), https://doi.org/10.3389/fphy.2022.884053
[3] M.A. May, N. Barré, K.K. Kummer, M. Kress, M. Ritsch-Marte and A. Jesacher: Fast holographic scattering compensation for deep tissue biological imaging. Nature Communications 12, 4340 (2021), https://doi.org/10.1038/s41467-021-24666-9
[4] M.A. May, K.K. Kummer, M-L. Edenhofer, J. L. Choconta, M. Kress, M. Ritsch-Marte, and A. Jesacher: Simultaneous scattering compensation at multiple points in multi-photon microscopy. Biomedical Optics Express 12, 12, 7377-7387 (2021), https://doi.org/10.1364/BOE.441604
[5] Molly A. May, Martin Bawart, Michiel Langeslag, Stefan Bernet, Michaela Kress, Monika Ritsch-Marte, and Alexander Jesacher, "High-NA two-photon single cell imaging with remote focusing using a diffractive tunable lens," Biomed. Opt. Express 11, 7183-7191 (2020), https://doi.org/10.1364/BOE.405863
[6] Martin Bawart, Molly A. May, Thomas Öttl, Clemens Roider, Stefan Bernet, Michael Schmidt, Monika Ritsch-Marte, and Alexander Jesacher, "Diffractive tunable lens for remote focusing in high-NA optical systems," Opt. Express 28, 26336-26347 (2020), https://doi.org/10.1364/OE.400784



