New antifungal strategies: structure and function of NFAP
FWF Lise Meitner Program (M 1776-B20)
Applicant: László Norbert Galgóczi (in scientific publications: László Galgóczy)
Co-Applicant: Florentine Marx
December 2014 - November 2016
Summary
The increased incidence of severe fungal infections and the fast development of drug resistant filamentous fungi causing mycoses, plant infections or damage of cultural heritages strongly demand for the development of new antifungal strategies. The NFAP from the mold Neosartorya fischeri (class Ascomycetes) is a member of the small, cysteine-rich, cationic and highly stable antifungal proteins. NFAP shows remarkable antifungal activity against numerous filamentous fungi. Features of NFAP render it exceptionally suitable as potential commercial preservative, bio-pesticide and drug against molds. A detailed study of the structure, structure-function relationship and potential targets of NFAP in sensitive fungi are still missing, although they are essential prerequisites to improve NFAP efficacy by drug design. This project focused on the investigation of these aspects.
We developed a novel Penicillium chrysogenum-based expression system, which allows cost-effective bulk production of cysteine-rich antifungal proteins for structural and functional analyses in a microorganism that is generally recognized as safe by the US Food and Drug Administration. From an economic view, the generation of high yields of antifungal proteins by fungal fermentation is more cost-effective than protein synthesis and our system could be easily adapted by the industry. By using this system, we resolved the compact solution structure of the highly stable and antifungal active NFAP. We further identified the “essential” structural determinants for the formation of the antifungal active compact structure of the NFAP, which allows to improve the antifungal activity and target-specificity of this bio-molecule by rational protein design or the synthesis of antifungal peptides. Target screening experiments revealed that NFAP might disturb mitochondrial function in sensitive fungi. This finding provides a potential new drug-target candidate for the pharmaceutical industry. As a “spin-off” result, we isolated and characterized a new cysteine-rich antifungal protein (NFAP2) from Neosartorya fischeri exhibiting high anti-yeast activity against clinically relevant human pathogenic Candida species. Thus, NFAP2 represents a highly interesting and promising antifungal compound for the development of new anti-yeast strategies. Our achievements regarding NFAP in this project will be supportive for the detailed analysis of NFAP2 in the near future.
Our results significantly contribute to the understanding of the mode of action and structure-function relation not only of NFAP but - considering the structural similarity - also of other cysteine-rich antifungal proteins in general, and pave the way for the development of new antifungal strategies.
The award of the Lise Meitner Program allowed the applicant and project leader László Galgóczi to refine his professional skills in science and supported his future academic career as follows: (i) The execution of the project fostered the continuation of the intensive studies to unravel the mechanistic function, and structure-activity relation of NFAP and NFAP2 at the University of Szeged based on the achieved results and acquired laboratory skills and methods. (ii) The benefits and results of the project enabled László Galgóczi to become a group leader and establish his own research group, Antifungal Proteins and Peptides Group at the University of Szeged with the support of two awarded follow-up grants, Postdoctoral Excellence Program (PD 120808) of the Hungarian National Research, Development and Innovation Office (NKFIH), and the bilateral FWF-NKFIH funded project with the co-applicant Florentine Marx from the Medical University of Innsbruck (I3132-B21; ANN 122833) (Raised Funding). (iii) The award of the Lise Meitner Program represented a significant step in László Galgóczi's research career to launch his application for habilitation in microbiology at the University of Szeged.
Within the Lise Meitner Program, the Bachelor student Simon Pöttinger from the Fachhochschule Gesundheit, Innsbruck (Austria), successfully completed and defended his thesis entitled "Expression des antifungalen Proteins AFP in Penicillium chrysogenum" in 2015. László Galgóczi was also involved in ongoing projects of the host lab and in the co-supervision of master and doctoral students. Results were disseminated in the frame of Biocenter lecture seminar series, and weekly in Friday morning meetings of the Applied Mycology group at the Medical University of Innsbruck. These activities further strengthened his teaching and supervisory skills.
The co-applicant Florentine Marx further strengthened her cooperative and scientific contact with the applicant László Galgóczi and with the international collaborators involved in this project (Collaborations). The continuation of the scientific cooperation of Florenine Marx with László Galgóczi is reflected by the award of the afore mentioned bilateral FWF-NKFIH funded project (I3132-B21; ANN 122833).
The joint efforts of all collaborators involved in this project is reflected in (i) two published and one submitted peer-reviewed papers in internationally renowned journals and two further manuscripts that are in preparation (Publications); (ii) the presentation of the elaborated results at several national and international conferences and meetings (Presentations); and (iii) the organization of two mini-symposia in 2015 and 2016, where internationally renowned scientific experts in the multidisciplinary fields of structural biology, biochemistry, mycology and biotechnology took part (Organization Mini-Symposia).
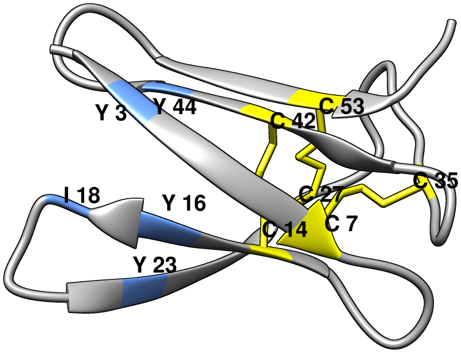
Fig.1: Predicted tertiary structure of Neosartorya fischeri NRRL 181 antifungal protein (NFAP). The hydrophobic core constituting amino acids are indicated by light blue. Cysteines and disulphide bridges are marked with yellow and yellow line.
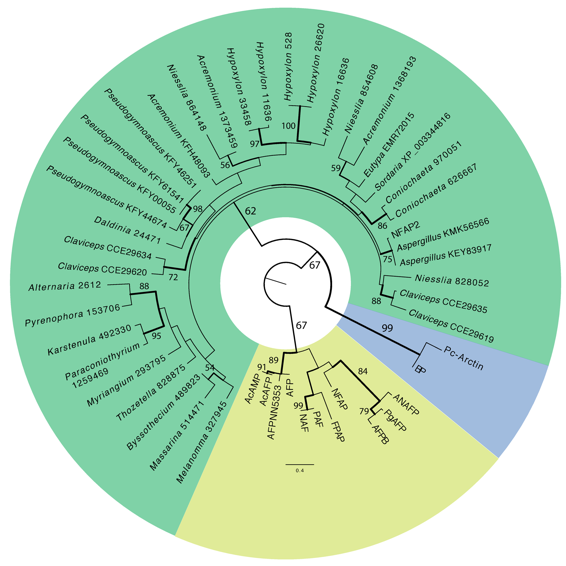
Fig.2: Phylogenetic relationship betweeen cysteine-rich antifungal proteins secreted by filamentous Ascomycetes. Yellow: Isolated and characterized Penicillium chrysogenum antifungal protein (PAF) homologs, Blue: Isolated and chracterized Penicillium brevicompactum 'bubble' protein (BP) homologs, Green: Neosartorya fischeri NRRL 181 antifungal protein 2 (NFAP2) and its putative homologs.



