Wachstumsfaktoren im Nervensystem
Wachstumsfaktoren und ihre Bindungsstellen (Rezeptoren) an Nerven- und Gliazellen spielen eine herausragende Rolle während der Entwicklung, bei neuroplastischen Veränderungen im Erwachsenenalter und bei verschiedenen neurologischen Erkrankungen. Wir forschen am Institut für Neuroanatomie über die Fibroblasten-Wachstumsfaktoren (FGFs), die spezifische Membranrezeptoren (FGF-Rezeptor-Tyrosin-Kinasen) aktivieren, deren intrazelluläre Signal- und Transportwege in unserem Labor primär anhand von neuronalen und glialen Zellkultur-Modellen untersucht werden. Für eine weitergehende Lektüre zur Rolle tropher Faktoren im Rahmen peripherer Nervenregeneration oder nach Querschnittsläsion empfehlen wir ein Sachbuch des Institutsleiters, das 2023 im Springer-Verlag erschienen ist.
----------------------------------------------------------------------------
Growth Factors in the Nervous System
Fibroblast growth factors (FGFs) are important for the development and repair of the nervous system. Stimulation of FGF receptors (FGFR) promotes neurogenesis, neuronal protection, axonal regeneration and remyelination in the injured nervous system. Our research focuses on the signalling and transport of FGFR type 1, the predominant FGF receptor in the nervous system (Csanaky et al. 2019). The inhibition of endocytosis and enhanced recycling of this receptor tyrosine kinase exert a significant influence on neuronal survival and axon outgrowth (Hausott et al. 2019).
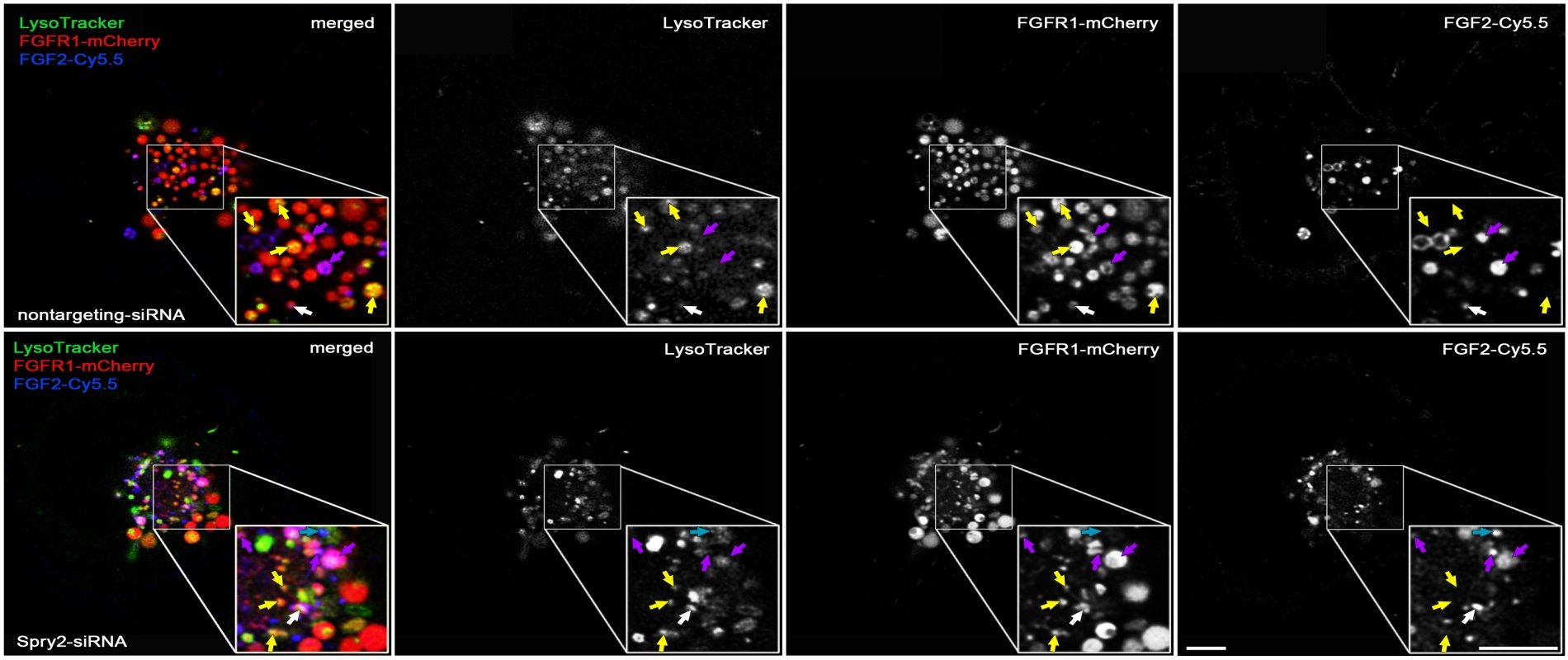
Among the negative feedback inhibitors of FGFR-induced signalling are the Sprouty proteins that comprise a family of four homologous molecules. Down-regulation of Sprouty2 and Sprouty4 promotes recovery from mechanical, vascular or excitotoxic brain lesions. Applying three different in vivo lesion models, we have demonstrated that the reduction of Sprouties in neurons or glial cells improves neuronal survival and axonal regeneration in the central and peripheral nervous system.
We have shown that primary sensory neurons dissociated from heterozygous Sprouty2 knock-out mice exhibit elevated MAP kinase (ERK) activity and enhanced axon outgrowth in response to nerve injury. Following sciatic nerve crush, significantly more myelinated axons regenerate in mice with reduced Sprouty2 levels, accompanied by faster recovery of function and increased expression of GAP-43 (Marvaldi et al. 2015). We also investigated a combined approach to promote long-distance axon growth in primary neuronal cell culture: dual-interference with Sprouty2 and PTEN, an inhibitor of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Our results clearly show that their simultaneous knockdown in neurons promotes axon elongation more strongly than the knockdown of each molecule individually (Jamsuwan et al. 2020).
With regard to the CNS, the injection of siRNAs against Sprouties into rat brains reduces the lesion size in response to endothelin-induced vasoconstriction (a model for stroke, Klimaschewski et al. 2016). Secondary brain damage is also significantly diminished in mice with reduced Sprouty2/4 levels. In response to kainate-induced excitotoxicity in the hippocampus, neuronal survival and reactive astrogliosis are enhanced in heterozygous Sprouty2/4 knock-outs as compared to their wild-type littermates (Thongrong et al. 2016).
Furthermore, Sprouty2 is a key regulator of glial tumour formation in the brain. Elevated Sprouty2 levels and epidermal growth factor receptor (EGFR) amplification are striking features of glioblastoma (GBM) and correlate with reduced patient survival. Sprouty2 enhances EGFR induced signaling through inhibition of ubiquitination and endocytosis of EGFR. Levels of FGF receptor type 1 (FGFR1) are increased in GBM as well. Knockdown of Sprouty2 dramatically inhibits glioblastoma growth. Ubiquitination of FGFR1 is decreased (in contrast to EGFR) and receptor endocytosis enhanced. ERK, AKT and PLC dependent signaling pathways are activated followed by premature S-phase entry. This results in DNA damage, cytotoxicity and disappearance of the tumor (Park et al. 2018, Hausott et al. 2019). On the other hand, overexpression of Sprouty2 limits FGF signaling in glioma cells by reducing FGFR1 levels and binding of PLCγ1 to the receptor (Hausott et al. 2024).
Taken together, treatment with FGFs and interference with Sprouties may provide novel therapeutic strategies to increase and prolong growth factor signalling in the lesioned peripheral nervous system and in the diseased brain.
For further information please see our review articles on the role of Sprouties (Hausott and Klimaschewski 2018) and FGFs in the nervous system (Klimaschewski and Claus 2021).
Institute of Neuroanatomy

Prof. Dr. med. Lars P. Klimaschewski
Institute of Neuroanatomy
Muellerstrasse 59
6020 Innsbruck, Austria

Prof. Dr. med. Lars P. Klimaschewski
Institute of Neuroanatomy
Muellerstrasse 59
6020 Innsbruck, Austria