Research on Ether Lipid Metabolism Division of Biological Chemistry, Biocenter, Medical University of Innsbruck
Katrin Watschinger, Markus Keller, Gabriele Werner-Felmayer, Georg Golderer, Ernst R. Werner
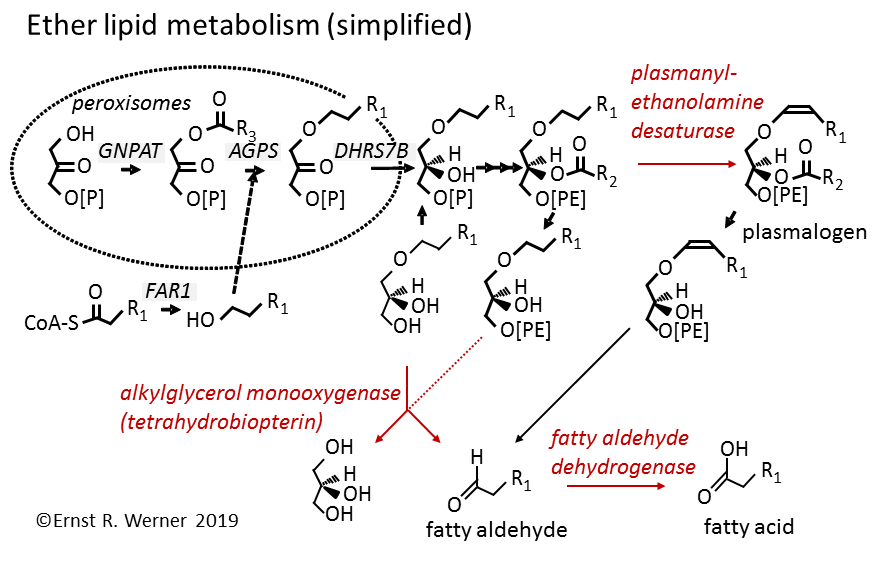
Building on 40 years of pteridine research in the institute, we are currently focusing on ether lipid metabolism. Degradation of ether lipids requires alkylglycerol monooxygenase, an enzyme dependent on the cofactor tetrahydrobiopterin. Tetrahydrobiopterin is structurally related to the vitamins folic acid and riboflavin, but is synthesized in the animal and human body. It and is required for five specific hydroxylation reactions. These include the conversion of phenylalanine to tyrosine by phenylalanine hydroxylase, the key step in the degradation of the essential amino acid phenylalanine. Tetrahydrobiopterin is currently used to treat phenylketonuria, a rare inherited human disease affecting phenylalanine hydroxylase.
In 2010 we assigned a sequence to alkylglycerol monooxygenase, 46 years after its first description. We then showed that knockdown of alkylglycerol monooxygenase had a profound impact on the lipidome of macrophage-like cells. Manipulation of tetrahydrobiopterin biosynthesis led to corresponding changes in the lipidome, thus establishing for the first time a functional connection of tetrahydrobiopterin to lipid metabolism of an intact cell.
Fatty aldehyde dehydrogenase, the subsequent enzyme in ether lipid metabolism, causes a severe rare inherited disease when its function is lost, the Sjögren Larrson Syndrome. We characterized by biochemical and structural methods the human fatty aldehyde dehydrogenase and found that a special additional structural feature of this enzyme, a gatekeeper helix, is responsible for its specificity to fatty aldehydes.
A current focus is the characterization of plasmanylethanolamine desaturase, which introduces the crucial double bond into plasmalogens, major glycerophospholipids in our brains and immune cell membranes. We developed novel assays to characterize this reaction and could find the gene (TMEM189, PEDS1) encoding this enzymatic activity.



