Neuroanatomy
Research Focus
General Facts
Research
Selected Publications
Collaboration
Keywords: Plasticity; Regeneration; Degeneration; Survival; Proliferation; FGF; Sprouty; Signalling; Endosomal Transport; Neurotrophic Factors
Research (ÖSTAT Classification) : 301405, 301402, 301403, 301407
Research Focus
Our laboratory investigates the role of growth factors in the nervous system under normal and pathological conditions. We aim to modify signalling pathway components downstream of growth factor receptors in neurons or glia that may act as pharmacological targets, in order to activate pro-survival and pro-regenerative mechanisms in the diseased nervous system. Our research focuses on the signalling and transport of fibroblast growth factor receptor type 1 and on its negative feedback inhibitor, Sprouty 2.
General Facts
The Institute of Neuroanatomy offers lectures and seminars in functional as well as comparative neuroanatomy for MD and PhD students. We primarily investigate the morphological consequences of growth factor signalling and receptor tyrosine kinase transport in the nervous system. Using mainly cellular methods in combination with high-resolution imaging, we study fundamental neurobiological phenomena such as axon outgrowth, nerve regeneration and glial proliferation in collaboration with local and international research groups.
Research
Fibroblast growth factors (FGFs) are important for the development and repair of the nervous system. Stimulation of FGF receptors promotes neurogenesis, neuronal protection, axonal regeneration and remyelination in the injured nervous system. Our research focuses on the signalling and transport of FGFR type 1, the predominant FGF receptor in the nervous system (Csanaky et al. 2019). The inhibition of endocytosis and enhanced recycling of this receptor tyrosine kinase have a significant influence over neuronal survival and axon outgrowth (Hausott et al. 2019).
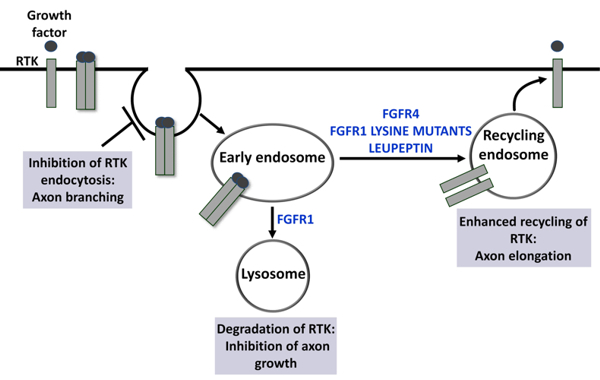
Fig. 1: In response to growth factor activation, receptor tyrosine kinases (RTKs) undergo endocytosis and are sorted into lysosomes for degradation or recycled back into the plasma membrane. FGF receptor type 1 (FGFR1) is rapidly sorted into lysosomes for degradation, whereas FGFR4 is predominantly recycled. These differences in endocytic transport are determined by different numbers of lysine residues acting as ubiquitination sites in the intracellular domain. The protease inhibitor leupeptin enhances recycling of FGFR1 and promotes elongative axon growth. Furthermore, lysine mutants of FGFR1 with enhanced recycling capabilities strongly promote elongative axon growth. By contrast, the inhibition of FGFR1 endocytosis inhibits axon elongation and enhances axonal branching.
Among the negative feedback inhibitors of FGFR1-induced signalling are the Sprouty proteins, which comprise a family of four homologous molecules. Down-regulation or knock-out of Sprouty 2 and 4 promotes recovery from mechanical, vascular or excitotoxic brain lesions. Applying three different in vivo lesion models, we have demonstrated that the reduction of Sprouties in neurons or glial cells improves neuronal survival and axonal regeneration in the central and peripheral nervous system.
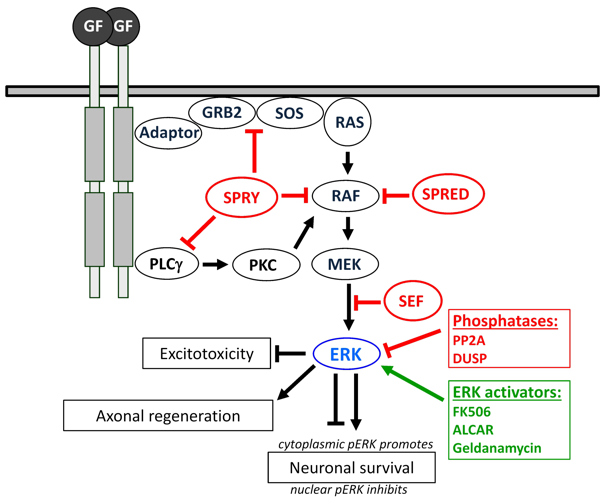
Fig. 2: The RAS/RAF/MEK/ERK pathway in neurons: Upon growth factor (GF) activation, FGFR autophosphorylates and recruits an adaptor (FRS2) followed by GRB2, which interacts with the guanine nucleotide exchange factor SOS that triggers the release of GDP from RAS. Activated RAS subsequently recruits RAF to the membrane. Active RAF acts on the dual-specificity kinase MEK, which phosphorylates ERK. The dimerised form of ERK exerts a variety of post-transcriptional as well as nuclear effects by phosphorylation of transcription factors. The RAS/ERK pathway is tightly regulated by several phosphatases including PP2A and DUSPs. Furthermore, signalling modulators, such as Sprouty (SPRY), SPRED and SEF, inhibit ERK signalling upstream and downstream of RAS. Activating drugs, such as FK506, ALCAR and Geldanamycin, enhance ERK signalling. Increased pERK levels promote axon regeneration and neuronal survival in response to excitotoxicity. Down-regulation of ERK negative feedback inhibitors, such as SPRY, or treatment with ERK activators, such as FK506, has also been demonstrated to promote nerve regeneration.
We have shown that primary sensory neurons dissociated from Sprouty 2 knockout mice exhibit elevated MAP kinase (ERK) activity and enhanced axon outgrowth in response to nerve injury. Following sciatic nerve crush, significantly more myelinated axons regenerate in Sprouty 2+/- mice, accompanied by faster recovery of function and increased expression of GAP-43 (Marvaldi et al. 2015). We also investigated a combined approach to promote long-distance axon growth in culture: dual-interference with Sprouty 2 and PTEN, an inhibitor of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Our results clearly show that their simultaneous knockdown in neurons promotes axon elongation more strongly than the knockdown of each molecule individually (Jamsuwan et al. 2020).
With regard to the CNS, the injection of siRNAs against Sprouties into rat brains reduces lesion size in response to endothelin-induced vasoconstriction (a model for stroke, Klimaschewski et al. 2016). Secondary brain damage is also significantly diminished in mice with reduced Sprouty 2/4 levels. In response to kainate-induced excitotoxicity in the hippocampus, neuronal survival and reactive astrogliosis are enhanced in heterozygous Sprouty 2/4 knockouts by comparison with their wild-type littermates (Thongrong et al. 2016).
Furthermore, Sprouty 2 is a key regulator of tumour formation in the brain (Park et al. 2018). It is up-regulated in malignant gliomas and this correlates with reduced survival in patients. Knockdown of Sprouty 2 dramatically inhibits glioblastoma growth and increases EGF-induced ERK and AKT activation concomitant with premature S-phase entry of tumour cells. Consistent with these findings, DNA damage response and cytotoxicity are increased.
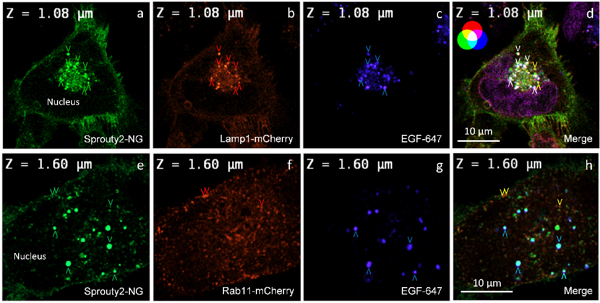
Fig. 3: Sprouty 2 is found at the plasma membrane and in the endosomal system of glial tumour cells. Fluorescence microscopy (two confocal planes are shown) demonstrates co-localisation of Sprouty 2 fused to NeonGreen (NG) with Lamp1-mCherry (a-d) or Rab11-mCherry (e-h) in U251 cells treated with fluorescently labelled EGF-647 in blue for 30 minutes (arrowheads indicate various endosomal vesicles with colocalised fluorescence).
Taken together, interference with Sprouties may provide a novel therapeutic strategy to increase and prolong growth factor signalling in the lesioned peripheral nervous system and in the diseased brain.
Selected Publications
- Csanaky, Katalin; Hess, Michael W.; Klimaschewski, Lars: Membrane-Associated, Not Cytoplasmic or Nuclear, FGFR1 Induces Neuronal Differentiation. CELLS. 2019; 8(3); 243. doi: 10.3390/cells8030243
- Hausott, B.; Klimaschewski, L.: Sprouty2-a Novel Therapeutic Target in the Nervous System? MOLECULAR NEUROBIOLOGY. 2019; 56(6); 3897-3903. doi: 10.1007/s12035-018-1338-8
- Hausott, Barbara; Foerste, Alexandra; Zach, Fabian; Mangger, Stefan; Haugsten, Ellen Margrethe; Klimaschewski, Lars: Endocytosis and Transport of Growth Factor Receptors in Peripheral Axon Regeneration: Novel Lessons from Neurons Expressing Lysine-Deficient FGF Receptor Type 1 in vitro. ANATOMICAL RECORD. 2019; 302(8); 1268-1275. doi: 10.1002/ar.24120
- Hausott, Barbara; Klimaschewski, Lars: Promotion of Peripheral Nerve Regeneration by Stimulation of the Extracellular Signal-Regulated Kinase (ERK) Pathway. ANATOMICAL RECORD. 2019; 302(8); 1261-1267. doi: 10.1002/ar.24126
- Hausott, Barbara; Park, Jong-Whi; Valovka, Taras; Offterdinger, Martin; Hess, Michael W.; Geley, Stephan; Klimaschewski, Lars: Subcellular Localization of Sprouty2 in Human Glioma Cells. FRONTIERS IN MOLECULAR NEUROSCIENCE. 2019; 12(S); 73. doi: 10.3389/fnmol.2019.00073
Collaborations
- Dr. P. Claus, Center for Anatomy, Hannover Medical School, Germany
- Dr. E. Haugsten, Department of Biochemistry, Institute for Cancer Research, Oslo, Norway
- Dr. S. Gajovic, Laboratory for Regenerative Neuroscience, University of Zagreb, Croatia
- Dr. P. Krejčí, Department of Biology, Masaryk University Brno, Czech Republic
 Univ.-Prof. Dr.med.univ. Lars Klimaschewski
Univ.-Prof. Dr.med.univ. Lars Klimaschewski
Director
Contact:
Müllerstrasse 59
6020 Innsbruck
Austria
Email: lars.klimaschewski@i-med.ac.at
Phone: +43 512 9003 71160
https://www.i-med.ac.at/neuroanatomy



