Hygiene and Medical Microbiology
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Infectious diseases, hygiene, immunity, fungal pathogens, antifungal resistance, HIV, dendritic cells, complement, N-chlorotaurine, nosocomial infections, public health
Research (ÖSTAT Classification) : 303026, 303002, 303015, 303013, 303020
Research Focus
Understanding Infections: From Pathogenesis to Diagnosis
- The tasks of the Institute of Hygiene and Medical Microbiology (HMM) comprise research, teaching, laboratory diagnosis of infectious diseases, environmental, hospital and technical hygiene as well as public health.
- Scientific activities cover fungal pathogenicity, antifungal resistance, virulence factors, molecular mechanisms of host pathogen interaction including the complement system, basic immunological research, antimicrobial agents and the prevention of nosocomial infections.
- HMM seeks to prevent illness and death from targeted infectious disease threats through research and the translation of scientific information into real-world, practical applications, policies, and solutions (Fig. 1).
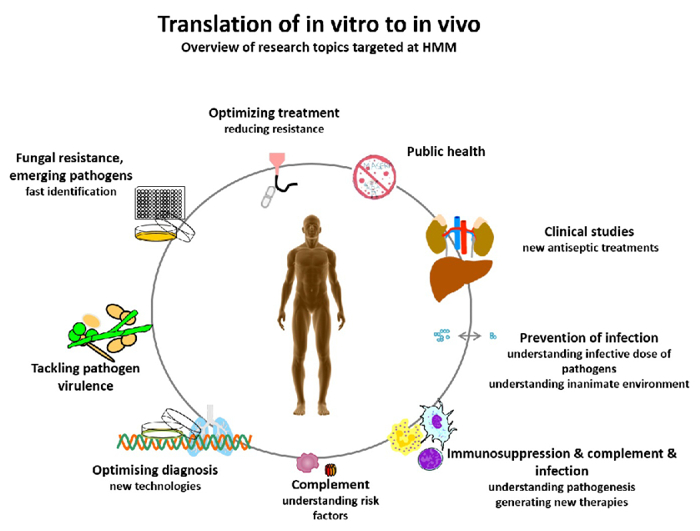
Fig. 1: Translation of in vitro to in vivo – overview of research questions targeted at HMM
General Facts
Infectious diseases are becoming one of the most frequent causes of death in the world; at present, we face bacteria and fungi that are developing resistance to antibiotics and antimycotics as well as the fact that an increasing number of emerging pathogens are spreading worldwide. Understanding the biological principles underlying the mechanisms by which infectious agents adapt and undermine the defence mechanisms of a host is critical to fighting diseases. HMM conducts basic and translational research into molecular mechanisms of pathogenesis of bacterial, viral, or fungal infections and strategies for prophylaxis and treatment. HMM’s mission is to coordinate and strategically align translational infection research with the aim of developing new diagnostic, preventive and therapeutic methods to treat infectious diseases. To achieve this, HMM has formed thematic translational units of scientists, each dedicated to one specific pathogen or infectious disease. HMM is one of the largest diagnostic microbiology laboratories in Austria and has an average sample throughput of 250,000 specimens per year. It is associated with all the major hospitals in Tyrol, and therefore holds a key position in the diagnostic laboratory landscape of Austria. The working groups of Reinhard Würzner, Doris Wilflingseder and Wilfried Posch cover the topic “Exploiting immune response”, the working groups of Michaela Lackner and Cornelia Lass-Flörl with Cornelia Speth and Ulrike Binder investigate fungal infections. “N-chlorotaurin” is the main research focus of Markus Nagl, and Peter Kreidl covers the topic of public health. Astrid Mayr is connected with the CD Laboratory. The mission of HMM is to bridge the gap between basic and translational research into microbial pathogenesis (Fig. 2).
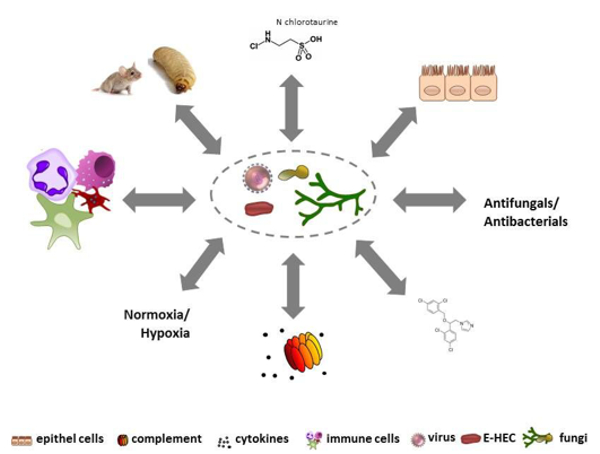
Fig. 2: Identifying underlying networks contributing to infectious diseases
Christian Doppler Laboratory for Invasive Fungal Infections
In 2015, a “Christian Doppler Laboratory for Invasive Fungal Infections” was set up. Within the estimated 2 million fungal species on earth, around 600 cause diseases in humans. The most significant are Candida, Aspergillus, Mucorales and Cryptococcus. Fungal infections are increasing and they are associated with excessive morbidity and mortality (Fig. 3). Over 300 million people are acutely or chronically infected, with the results of death, long term illness and reduced work capacity. The reasons for emergence are probably multifactorial, e.g. the advent of medical progress, the successful application of immunosuppression in transplant patients and the use of immunomodulatory agents to treat various diseases from cancer to rheumatoid arthritis.
CD Fungus is attempting to unravel the scientific questions raised, by implementing 3 modules that will ultimately advance our understanding of fungal pathology, improve diagnosis and treatment of mucormycosis, and enhance patient outcome and safety in terms of prevention of nosocomial and hospital-related infections.
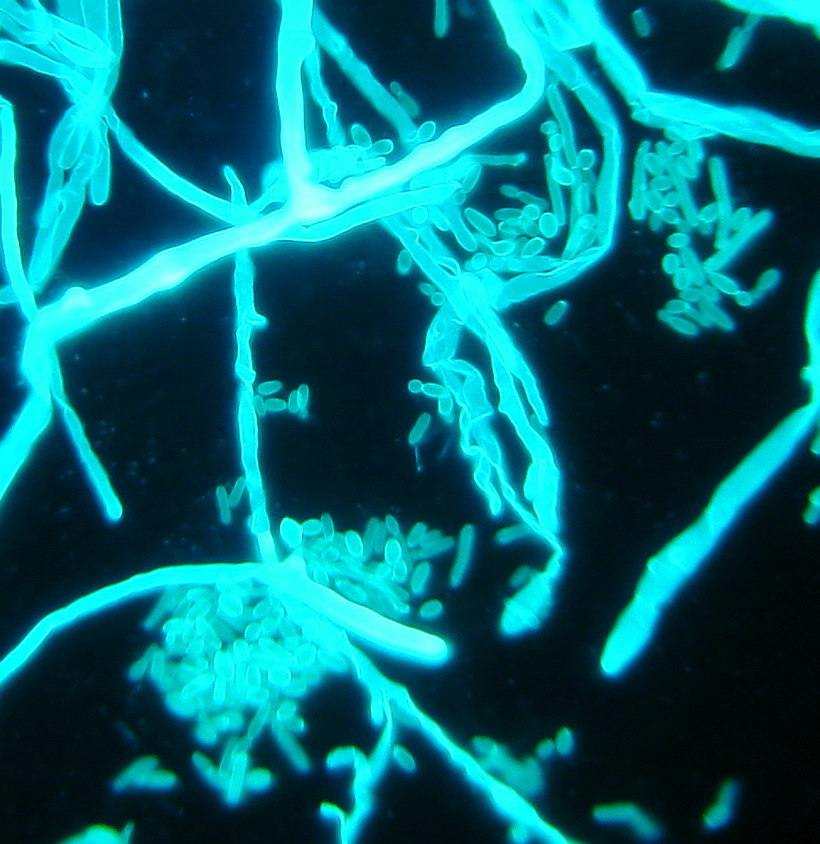
Fig. 3: Invasive fungal lung infection displaying hyphae (mucor species) and yeast cells
Research
Exploiting Immune Response
Complement is a tightly controlled arm of the innate immune system, which facilitates phagocytosis and the killing of invading pathogens. Factor H (FH) is the main fluid-phase inhibitor of the alternative pathway. Many pathogens interact with complement to evade the immune system of the host and especially to evade complement; some hijack FH from the host and protect themselves from complement-dependent killing. Candida albicans is a clinically significant, opportunistic yeast that displays different FH-binding molecules on its cell surface, which allow complement evasion. One such FH-binding molecule is the transmembrane protein “high-affinity glucose transporter 1” (Hgt1p) involved in glucose metabolism, which we will study in greater detail in the future.
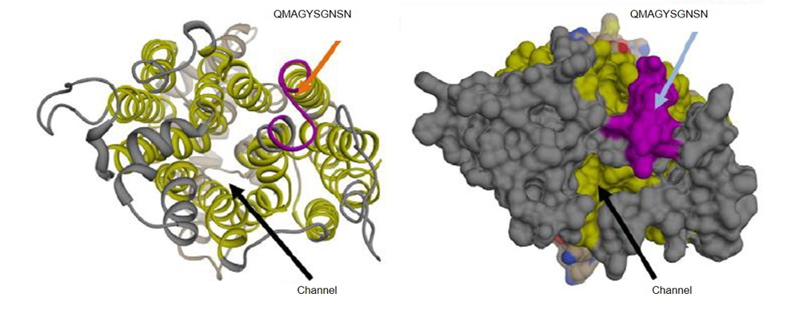
Fig. 4: 3D schematic representation of Hgt1p. The antibody anti-Hgt1p generated in this project recognises an epitope exposed in an extracellular portion of C. albicans cell membrane (upper arrow); the glucose channel is also clearly visible (lower arrow).
Another focus is on dendritic cells (DCs) – the most important antigen-presenting cells in the body – and their interaction with the complement system. The complement system is activated immediately after entry of pathogens such as HIV-1 and it marks the surface of the intruder in a process called opsonisation. Such marked pathogens are more recognisable to dendritic cells. Since HIV-1 is very well protected from attacks by the complement system, the virus is not spontaneously destroyed by this aggressive first immune defence but rather covered by permanently bound complement fragments. Thus, the actual binding domains of HIV-1 are hidden, which enables interaction with complement receptors (CR) on host cells such as DCs or macrophages when the virus enters the body.
By comparing complement-coated HIV-1 with HIV-2, we characterise molecules in cells that initiate an antiviral status and activation of the innate and adaptive immune response. These components are designed to make the virus more visible to cellular pattern recognition receptors. Further, the role of complement in the metabolic reprogramming of dendritic cells is being investigated as part of the newly approved “Cellular Basis of Diseases – CBD” graduate programme.
Highly differentiated cell culture models are also being developed by Wilflingseder’s group, in order to allow research into the initial interactions between various pathogens (HIV-1, Aspergillus, Chlamydia) in the acute phase of infection. The established human 3D mucous membrane model is very promising in this context. The in vitro system allows detailed analyses of HIV-1 and HIV-2 with molecules and enzymes of an intact, complex mucous membrane and the development of novel therapeutic approaches. The mucosal/immune model is funded with a grant from the National Institutes of Health (NIH). Together with our American colleague, Thomas J. Hope from Northwestern University in Chicago, the behaviour of HIV-neutralising antibodies in this human mucosal model will be investigated, in order to pave the way for new vaccination strategies against HIV.
The respiratory barrier/immune model has been optimised in recent years as part of an ÖNB project. This 3D model and differentiated apical-out lung organoids are applied in further studies using pathogenic fungi (in cooperation with CL-F.) or SARS-CoV-2 (project leader: WP > COVID19 project).
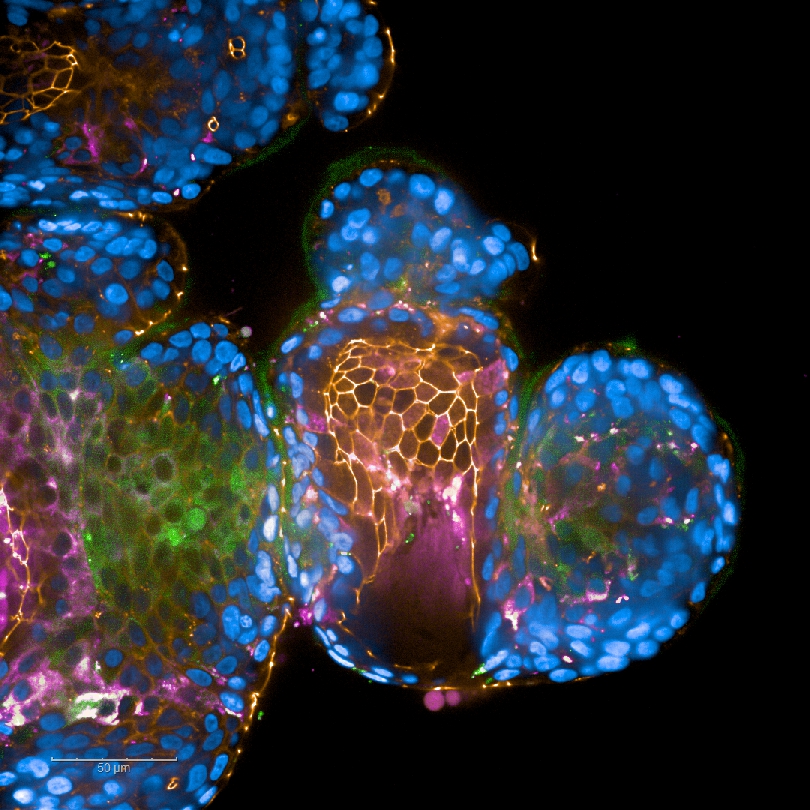
Fig. 5: HIV-1-infected DCs
The current coronavirus (COVID-19) pandemic has far-reaching consequences with unprecedented disruption to modern society from locking down much of the world, crashing economies and healthcare services, and more. The infectious agent causing COVID-19 is the virus SARS-CoV-2. Its extremely rapid spread within populations around the globe increases the need for understanding of the pathogenicity of the virus during entry via respiratory tissues as well as for testing of effective drugs or novel vaccination strategies. Recently, we developed optimised respiratory human 3D system model systems and lung organoids that mimic the upper and lower respiratory tract and that are therefore ideal for the investigation of SARS-CoV-2 infections. In addition, immune cells can be included in these respiratory models and relevant host-pathogen interaction scenarios can be investigated. We aim (i) to monitor the pathogenesis of SARS-CoV-2 during early transmission events and to characterise tissue and cellular damage, (ii) to investigate immune-mediated mechanisms in the early and late phase of infection, and (iii) to test possible treatment options using drugs already approved for other diseases. Our respiratory 3D tissue models and lung organoids will contribute to additional treatment options for COVID-19 patients and furthermore provide new knowledge of tissue damage and viral transmission.
In addition, other ongoing SARS-CoV-2 projects involve the development of a very sensitive ELISpot assay specific to SARS-CoV-2 for analysis of the T-cell-mediated immunity of COVID-19 patients and neutralisation assays to determine the neutralising capacity of antibodies in COVID-19 patient sera. In a collaborative work on wastewater-based epidemiology, we have designed quantitative, PCR-based assays to monitor SARS-CoV-2 levels in Tyrolean wastewater plants, in order to identify local COVID-19 clusters.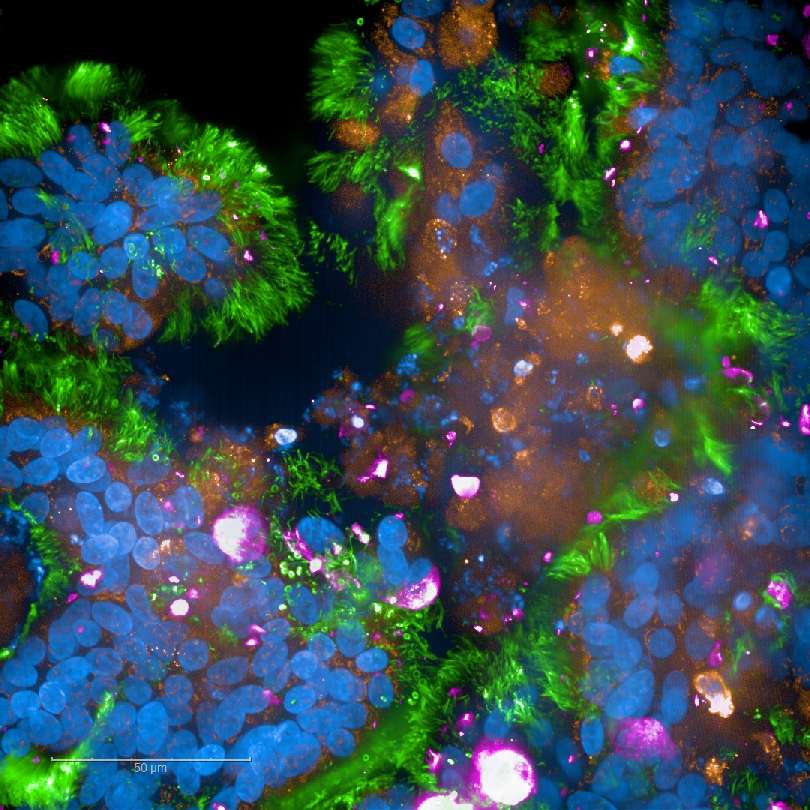
Fig. 6: SARS-CoV-2-infected respiratory tissue model
Therapy-resistant Fungal Infections
A disturbing and rapid increase in infections caused by antimycotic-resistant fungal pathogens is one major public health concern facing medicine today. Most severe and fatal cases result from healthcare-associated fungal infections, which are increasingly caused by Candida, Aspergillus and Mucorales. Hence, one significant focus is the investigation of azole and echinocandin resistance in yeasts and moulds and the discovery of new resistance mechanisms. Another major focus is on identifying the underlying mode of amphotericin B resistance in Aspergillus terreus. In this context, we are evaluating mitochondria as crucial modulators of polyene resistance. The mission of HMM is to bridge the translational gap between basic research and the development of novel antifungal drugs. HMM will support epidemiologic, translational and clinical studies to improve the management of fungal diseases.
Structure and Function-guided Development of Next-generation Azole Drugs
Understanding of the mechanism of acquired and intrinsic azole resistance in a wide range of fungal pathogens is therefore extremely relevant to the broad field of antifungal drug discovery. Our strategy is to decipher the molecular basis of azole resistance and to generate essential information for drug discovery and development.
The target of azole drugs is the cytochrome P450 enzyme lanosterol 14α-demethylase (LDM) (Fig. 7). For fungi, this is a key enzyme in the ergosterol biosynthesis pathway, catalysing the 3-step conversion of lanosterol into the fungus-specific sterol ergosterol. Azole drugs compete with the substrate lanosterol. Binding azoles to the ligand-binding pocket leads to depletion of ergosterol. Toxic intermediate products then accumulate and membrane fluidity and integrity are reduced. The consequence is the inhibition of fungal growth.
Our research focuses on measuring the impact and elucidating the structural basis of key AA substitutions in the ligand-binding pocket of LDMs on azole resistance. The substitution of such AAs can lead to a loss of key water-mediated hydrogen bond networks between the ligand and the ligand-binding pocket (Fig. 7). Hydrogen bond networks within the ligand-binding pocket are particularly important for binding short-tailed azoles (e.g. voriconazole). In addition, differences in triazole structure determine azole potency, toxicity and drug efficacy.
We used a platform based on Saccharomyces cerevisiae to elucidate the mechanism of innate and acquired azole resistance of various pathogenic fungi. Data from this heterologous expression model have provided a deeper understanding of azole resistance mechanisms. LDM crystal structures offer the opportunity for structure-guided development of a new generation of more effective, broad-spectrum azole drugs that have stronger binding affinities and improved specificity. By combining the structural and functional information with the screening tools obtained, we will greatly expedite the discovery and development of antifungals that effectively target the LDM in fungal pathogens and have low binding affinities for human LDM and liver drug detoxifying enzymes, thereby reducing drug toxicity.
Our aim is to establish a comprehensive and practical tool for the screening and discovery of novel broad-spectrum antifungals applicable in human medicine.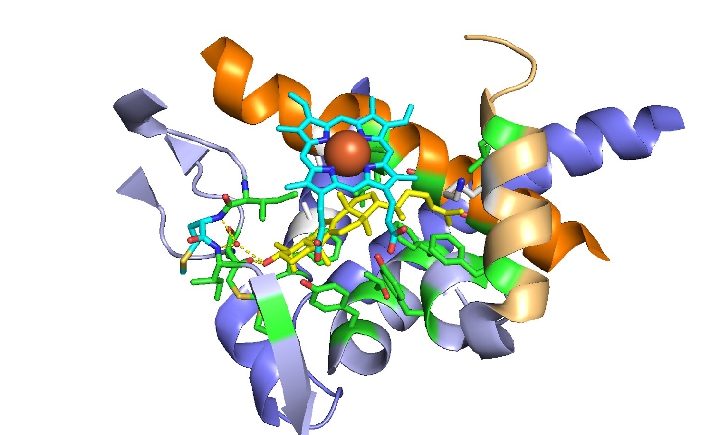
Fig. 7: Structural overview of the ligand-binding pocket of lanosterol 14-alpha demethylase (LDM) in the human CYP51 D231A H314 mutant catalytic domain interacting with lanosterol (yellow). The heme-domain is shown in light blue and the iron is shown as a red ball. Key amino acid residues that are important for ligand binding are shown in green. The water-mediated hydrogen bond network involving the OH of lanosterol is shown as a yellow dotted line.
N-chlorotaurine
N-chlorotaurine (NCT), a long-lived oxidant produced by activated human leukocytes, has been synthesised as sodium salt in our laboratory and is being investigated as a new antiseptic for the topical treatment of infections of multiple body regions, including sensitive ones.
Basic research currently comprises its microbicidal activity against viruses including SARS-CoV-2, multiresistant bacteria and fungi biofilms. Latest clinical investigations cover the bronchopulmonary system (confirmatory phase II inhalation of NCT in Covid-19 patients planned) as well as various other topical applications, such as to the skin and mucous membranes, the eye, the ear-nose-throat region, the urinary tract and organ abscesses. Advantages of NCT include high tolerability, a broad spectrum of activity against all strains of pathogens without inducing resistance, inactivation of virulence factors of pathogens, enhancement of microbicidal activity in the presence of body fluids and anti-inflammatory effects.
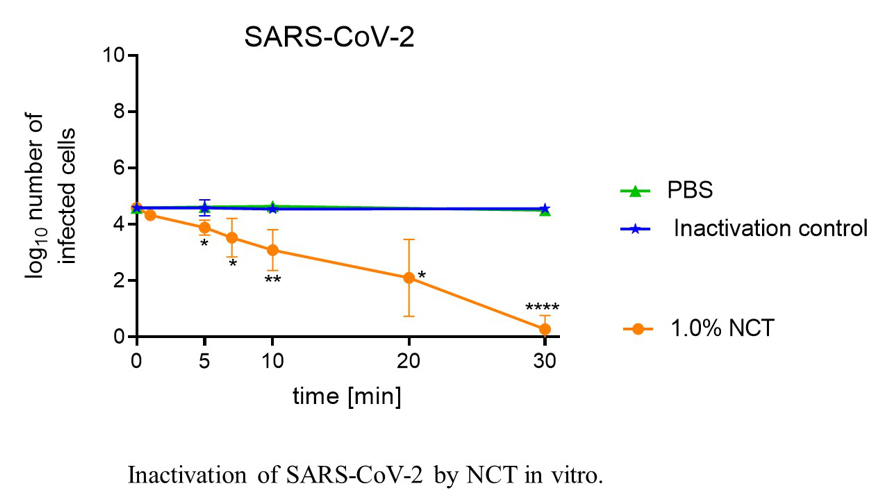
Fig. 8: Inactivation of SARS-CoV-2 by NCT in vitro
Laboratory Diagnostics, Hospital and Technical Hygiene
The HMM division fulfils its tasks in the detection and identification of pathogens that cause infections. These cover bacteriology, parasitology, mycobacteriology and mycology. The diagnostic laboratories are certified according to ISO 9001:2009. Special parts are controlled by external audits in accordance with §67 Austrian Medicines Law and FDA, Manufacturing and Product Quality division. Within the sector of hospital and technical hygiene (accredited under ISO/IEC 17025 and ISO/IEC 17020), guidelines for the prevention of infectious diseases are developed and controlled in line with the statutory requirements for technical facilities (e.g. disinfection machines).
Public Health
The World Health Organization identified vaccine hesitancy (VH) as one of the ten major global public health threats in 2019. VH is a growing, complex and context-specific problem in many European countries, and the goal of eliminating measles and rubella is at stake.
We must take measles elimination seriously, as the number of measles cases worldwide surged to nearly 870,000 in 2019 – the highest number reported since 1996 – and the number of deaths globally had increased nearly by 50% since 2016 to an estimated 207,500 deaths in 2019, a global average of one death every 2.5 minutes.
Although the incidence of many vaccine-preventable diseases has significantly decreased as a result of the previous and current lockdowns, we must expect outbreaks of these highly transmissible diseases (especially measles) in the future. This is mainly due to the administration of fewer vaccines during these difficult times and the resulting accumulation of susceptibles.
VH may also hamper the introduction of COVID-19 vaccines once they become available. For a number of years, we have provided undergraduate training in schools, to enhance health literacy and knowledge of vaccinations and to identify reasons for hesitancy. This will help with the tailoring of communication strategies and address concerns as well as hopefully increasing vaccination uptake to prevent future outbreaks of vaccine-preventable diseases and to move closer to the target of eliminating measles and rubella.
Selected Publications
- Kenno S, Speth C, Rambach G, Binder U,; Chatterjee S, Caramalho R, Haas H, Lass-Floerl C, Shaughnessy J, Ram S, Gow N, A. R.; Orth-Hoeller D, Wuerzner R: Candida albicans Factor H Binding Molecule Hgt1p-A Low Glucose-Induced Transmembrane Protein Is Trafficked to the Cell Wall and Impairs Phagocytosis and Killing by Human Neutrophils.
FRONTIERS IN MICROBIOLOGY. 2019; 9(S); 3319. - Bermejo-Jambrina M, Blatzer M, Jauregui-Onieva P, Yordanov TE, Hörtnagl P, Valovka T, Huber LA, Wilflingseder D, Posch W. Front Immunol: CR4 Signaling Contributes to a DC-Driven Enhanced Immune Response Against Complement-Opsonized HIV-1. eCollection 2020. doi: 10.3389/fimmu.2020.02010. 2020; 14;11:2010.
- Chandorkar P, Posch W, Zaderer V, Blatzer M, Steger M, Ammann CG, Binder U, Hermann M, Hörtnagl P, Lass-Flörl C, Wilflingseder D: Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus Sci Rep. doi: 10.1038/s41598-017-11271-4. 2017 Sep 14;7(1):11644.
- Bassetti M, Vena A, Bouza E, Peghin M, Munoz P, Righi E, Pea F, Lackner M, Lass-Flörl C: Antifungal susceptibility testing in Candida, Aspergillus and Cryptococcus infections: are the MICs useful for clinicians? Clin Microbiol Infect. 2020, 26:1024-1033.
- Cornely OA, et al.: Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. doi: 10.1016/S1473-3099(19)30312-3. 2019 Dec;19(12):e405-e421.
- Leiter H, Toepfer S, Messner P, Rabensteiner M, Gostner JM, Lackner M, Hermann M and Nagl M: Microbicidal activity of N-chlorotaurine can be enhanced in the presence of lung epithelial cells. Journal of Cystic Fibrosis. doi: 10.1016/j.jcf.2020.03.005. March 2020;
- Kreidl P, Breitwieser MM, Würzner R, Borena W.: 14-year-old Schoolchildren can Consent to Get Vaccinated in Tyrol, Austria: What do They know about Diseases and Vaccinations? Vaccines (Basel). doi: 10.3390/vaccines8040610. 2020 Oct 15;8(4):E610.
Selection of Funding
- EU, Horizon2020, Marie Sklodowska Curie Action European Joint Doctorate “CORVOS”, 860044, Complement regulation & variations in opportunistic infections. 2019-2023
- FWF P33510: HIV-C entflieht Restriktion, nicht Sensing in DCs. 2020-2024
- FWF P 34070: Treating SARS-CoV-2 infection in human 3D respiratory models. 2020-2024
- FWF P32329-B: Intrinsic azole resistance in mucormycetes. 2019-2023
- CD-Labor für Invasive Pilzinfektionen. 2015–2022
- Austrian Cystic Fibrosis self-regulating community‘Mukoviscidose HILFE Oberösterreich’, CF Austria (Cystische Fibrose Hilfe Österreich), Mukoviszidose Hilfe Wien, Niederösterreich and Nördliches Burgenland.
Collaborations
- Peter Garred, HOROS Guest Professor, Rikshospitalet, University of Copenhagen, Denmark
- Prof. Teunis B Geijtenbeek, Center of Infection and Immunity, Academic Medical Center, Amsterdam, The Netherlands
- Arnaud Moris, INSERM UMRS945, Infection et Immunité, UPMC, Paris, France
- Oliver Cornely, Studienzentrum Infektiologie, Universitätsklinik Köln, Deutschland
- Brian Monk, Department of Oral Sciences, Division of Health Sciences, University of Otago, Dunedin, New Zealand
- Doz. Dr. Carsten Schwarz, Christiane Herzog Zentrum Berlin/Charité-Universitätsmedizin Berlin, Berlin, Germany
 Univ.-Prof.in Dr.in med. Cornelia Lass-Flörl
Univ.-Prof.in Dr.in med. Cornelia Lass-Flörl
Director
Contact:
Schöpfstraße 41
6020 Innsbruck
Austria
Email: hygiene-bakteriologie@i-med.ac.at
Phone: +43 512 9003 70703
Fax: +43 512 9003 73700
www.i-med.ac.at/hygiene



