Clinical and Functional Anatomy
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Applied Anatomy, Ultrasound, Neuromonitoring, Human Embryology, Immunohistochemistry, Pelvic Organs, Cardiovascular System
Research (ÖSTAT Classification) : 301102, 301103, 301104, 301106, 301107, 301111, 301112, 301113, 301115
Research Focus
Our institute has two major research topics: applied anatomy and human embryology.
Applied Anatomy
Classical methods of preserving human tissue are used in combination with modern imaging, operative and biomechanical methods for research purposes. In order to be able to implement new (minimally-invasive) operations, techniques and imaging on body donors to science in a high-quality and realistic way, special methods such as a preservation based on alcohol/glycerine are used.
Human Embryology
A branch of anatomy, human embryology is a mainstay of descriptive as well as modern clinical and functional anatomy. This is because embryology correlates morphological structures with human development, function and possible dysfunctions in comparison with well-established animal models. For our research, human adult and human embryonic samples are examined microscopically. Various histological stainings as well as immunohistochemistry, in-situ hybridisation, proximity ligation assay, light fluorescence and electron microscopy are used and evaluated professionally. The establishment of 3D cell cultures from primary tumour material has been successfully added.
General Facts
The principal task of the Institute of Clinical and Functional Anatomy is the study and teaching of human anatomy and embryology. The aim of anatomy is to understand the normal structures and architecture of the organism, and the aim of embryology is to understand its normal development. Our research and teaching rely on an extensive body donation programme. We offer numerous lectures and courses in anatomy and embryology for MD students, PhD students and physicians in various disciplines. The research focus of our groups is on clinical applied anatomy, with a special emphasis on peripheral nerves, and on embryology, with an emphasis on the pelvic organs. Various macroscopic and modern microscopic techniques, including CT, MRI and US imaging, are applied.
Research
Applied Anatomy – Peripheral Nerves
Konschake, Moriggl
All our “Applied Anatomy” courses are delivered with the aim of developing new surgical approaches, improving modern imaging techniques and finding new applications for clinical practice (Fig. 1, Fig. 2).
Urinary and faecal incontinence are known complications after low anterior resection (LAR). Preserving autonomous pelvic nerves with no technical assistance is challenging. Our research is therefore investigating the technical feasibility of a new method for pelvic intraoperative neuromonitoring (pIONM). In twelve female pigs undergoing LAR, the pIONM included direct pelvic nerve stimulation and voltage measurement on the urinary bladder and the rectum, to identify efferent pelvic nerves in the surgical area. Immunohistochemistry of tissue samples obtained after direct nerve stimulation was used for autonomous nerve tissue verification using S100, VIP, DBH and TH antibodies. In two human body donors to science, the autonomous pelvic plexus was dissected and it was possible to display the position of the electrode. Smooth muscle contraction of the urinary bladder and/or the rectum as a result of direct stimulation of the innervating nerves was detectable with voltage measurement. The macroscopic contraction of both the urinary bladder and the rectum correlated with a change in voltage drop compared with the status before contraction. It was therefore possible to identify pelvic nerves in the surgical area.
With applied anatomical research in the field of endocrine surgery, we investigate special relationships between the location and blood supply of organs (e.g. thyroid, parathyroid), in order to minimise intraoperative risks of damage to peripheral nerves as well as vessels and organs, with all possible postoperative consequences for the patient: hypoparathyroidism remains one of the most common complications of thyroid surgery. Our research aims to improve understanding of the complexity of the blood supply and the localisation of the parathyroids in comparison with the two most important intraoperative landmarks: the inferior laryngeal nerve (ILN) and Zuckerkandl’s tubercle (ZT). 103 laryngeal compounds were examined. For intraoperative localisation, we defined a Cartesian coordinate system, with the ZT plane as the x-axis and the course of the inferior laryngeal nerve as y-axis. The inferior thyroid artery (ITA) provides the main supply to the parathyroids, whereas the superior thyroid artery provides a backup supply. 73.5 % of all parathyroids lie within 1 cm of the ILN and 1 cm cranial and 2.5 cm caudal to the ZT plane. Our described perimeters mark the most crucial areas during surgery and provide the surgeon with an anatomical map that shows areas in which special care is needed.
Furthermore, new, minimally invasive approaches for various peripheral nerve compression diseases are being investigated with the application of ultrasound, e.g. tarsal tunnel syndrome and carpal tunnel syndrome. As a result, patients receive a new, minimally invasive surgical method, in which decompression of the peripheral nerve can be achieved via a 2 mm approach only.
In the field of neurosonography, the main applications for clinical and preoperative practice are developed on body donors to science and on volunteers, in order to be able to apply the results to our patients immediately, e.g. in the field of interventional radiology and ENT as well as regional anaesthesia and pain therapy (Fig. 1, Fig. 2).
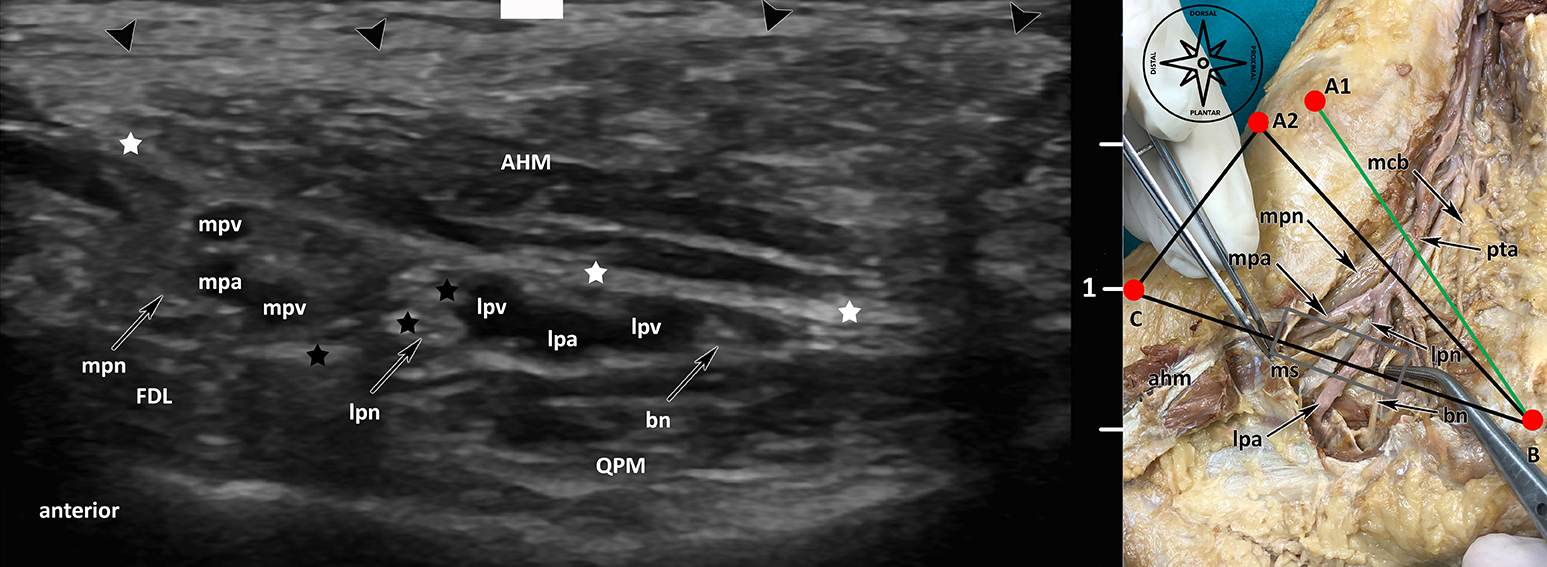
Fig. 1: Ultrasound visualisation of the terminal branches of the tibial nerve (lpn, mpn, bn) on [BC] line: lpn: lateral plantar nerve, mpn: medial plantar nerve, bn: Baxter’s Nerve, FHL: flexor hallucis longus muscle, QPM: quadratus plantae muscle, AHM: abductor hallucis muscle, mpv: medial plantar vein, lpv: lateral plantar vein, mpa: medial plantar artery, lpa: lateral plantar artery, black arrowheads: superficial layer flexor retinaculum, white stars: medial septum (deep fascia of AHM), black stars: medial septum (interfascicular septum).
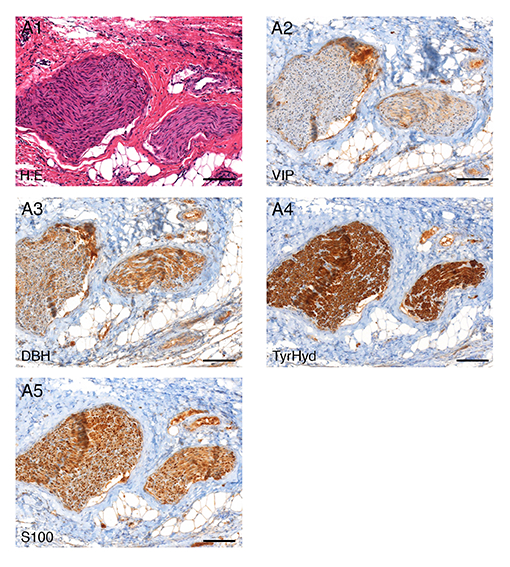
Fig. 2: Histology and immunohistochemistry verification of nerve tissue in the samples obtained from pigs: A1 H.E. staining, A2 vasoactive-intestinal-peptide (VIP) staining, A3 dopamine-ß-hydroxylase (DBH) staining, A4 tyrosine-hydroxylase (TyrHyd) staining, A5 S100 staining, 10x magnification.
Human Embryology
Fritsch, Pechriggl
Prenatal organogenesis of the urethra and mesodermal epithelial interactions in penile tissue of boys with and without hypospadias
Our aim is to understand the regulatory mechanisms during organogenesis of the human urethra and the determining gene and protein expression scores of patients with hypospadias, in order to provide strategies for selection of patients who would benefit from (neo)adjuvant hormonal therapy as well as surgical procedures. Human tissue was therefore screened by means of immunohistochemical and molecular methods, for qualitative and quantitative alterations with regard to epithelial, mesenchymal and stem cell proteins.
In our study, “The male urethra: Spatiotemporal distribution of molecular markers during early development”, we examined human embryonic/foetal specimens between the 6th and the 15th gestational week, by means of histomorphological methods. We were able to demonstrate that cranial to caudal differentiation of the urogenital sinus into urothelial epithelium begins before rupture of the cloacal membrane and becomes transitional over the whole length at weeks 8/9 (Fig. 3).
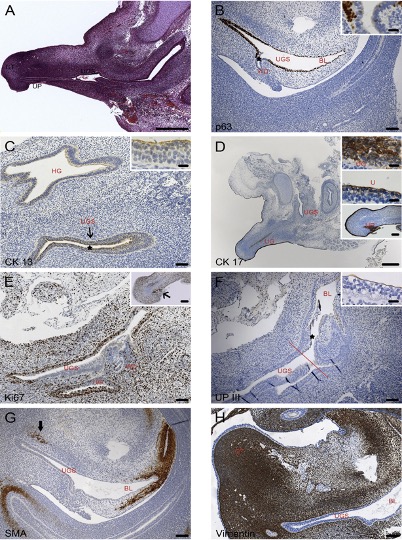
Fig. 3: Sagittal sections of pelvic organs of 8 and 9-week-old male embryos: (A) HE staining; (B – D and F) Immunohistochemical markers as labelled in the single pictures. BL: bladder; GT: genital tubercle; HG: hindgut; U: urethra; UG: urethral groove; UGS: urogenital sinus; UP: urethral plate; WD; Wolffian duct.
Furthermore, the urogenital sinus epithelium provides the Wolffian duct with p63-positive cells, leading to the suggestion that development of the male inner genitals requires a cellular stimulus by the UGS. CK 17 positive cells, which were described as stem cells in previous studies, were found in the urethral plate, the precursor of the distal urethral tube.
Fibroblast growth factors (FGFs) play a crucial role in early embryogenesis of the genital tubercle and are downstream targets of androgens during external genital development. In our study, “FGF8, FGF10 and FGF receptor 2 in the foreskin of children with hypospadias: an analysis of immunohistochemical expression patterns and gene transcription”, we focused on the expression patterns and transcription levels of FGF8, FGF10 and FGF receptor 2 (FGFR2) in patients with hypospadias by comparison with normal controls. Patients with hypospadias consistently showed aberrant (epi)dermal immunohistochemical staining patterns for FGF8/FGF10/FGFR2 with an unaltered expression of the relative mRNA, which represents a hint at important consequences of early embryological events for penile skin in hypospadias patients.
In addition to endodermal differentiation, we hypothesised that ectodermal surface epithelia morphogenesis is also involved in hypospadias. In our study, “Langerhans cells in hypospadias: an analysis of Langerin (CD 207) and HLA – DR on epidermal sheets and full thickness skin sections”, (epi)dermal sheets incubated with antibodies against Langerhans cells were prepared from skin samples of patients with and without hypospadias. Langerhans cells are present in normal frequency and morphology in the foreskin of hypospadias patients. This might suggest that patients with hypospadias are no different in consideration of their specific skin immunity, at least with respect to epidermal compartment.
History of Medicine
Brenner
In recent years, historians and one of our staff members reappraised the Nazi history of our institute. In this context, the origin of the corpses used for teaching and research during the Nazi era was investigated. It was found that the bodies came from an unusually broad range of sources: prisoners executed at Stadelheim Prison in Munich, prisoners of war from various camps, military personnel sentenced to death by martial courts, patients from a psychiatric hospital, and several bodies of Jewish Holocaust victims. The research also revealed an exchange between institutions: histological slides of Nazi victims had been provided by the Leipzig Institute of Anatomy.
Collaboration with Heart Surgery
Ischaemic cardiomyopathy is the deadliest killer worldwide and prognoses estimate an increase in prevalence in the coming decades. Current treatment strategies, such as pharmacotherapy or revascularisation, provide symptomatic relief and at best stop progression of the disease. Despite intensive research and some auspicious preclinical results, no regenerative therapy is available for routine clinical application.
Shockwaves are mechanical sound pressure waves. Shockwave therapy (SWT) has been used in routine clinical application for over 30 years, in order to detect kidney stones (lithotripsy). The regenerative effect of SWT was discovered coincidentally. SWT is therefore routinely used in the treatment of multiple orthopaedic pathologies and chronic non-healing wounds. Our group was able to demonstrate a regenerative effect of SWT in the ischaemic heart. In the recently published work, we were able to describe the exact mechanism whereby the mechanical stimulus of SWT is translated into a biological signal.
We were able to demonstrate that endothelial cells release exosomes, microvesicles of approximately 100 nm, on SWT. In various functional in vitro experiments, we were able, via the phosphorylation of AKT and ERK, to observe angiogenic and proliferative effects of released exosomes. The injection of SW-released exosomes in a murine myocardial infarction model results in increased ventricular function and decreased levels of fibrotic scar formation. Furthermore, we were able to identify microRNA 19a-3p as the exosomal cargo responsible. We demonstrated an angiogenic and proliferative effect of MiR19a-3p in multiple in vitro experiments; when administered in our murine myocardial infarction model, we observed a regenerative effect and increased ventricle function.
As a result of this and multiple preliminary works, we initiated the CAST-HF trial (Safety and Efficacy of Direct Cardiac Shockwave Therapy in Patients with Ischaemic Cardiomyopathy Undergoing Coronary Artery Bypass Grafting, ClinicalTrials.gov Identifier: NCT03859466). In this prospective, randomised trial, patients suffering from decreased left ventricular function (LVEF < 40%) are treated with SWT in addition to coronary artery bypass grafting surgery. Ventricular function is evaluated by means of cardiac MRI in a 12-month follow up period. By November 2020, 41 patients had been recruited to this study.
Selected Publications
- Burger F, Fritsch H, Zwierzina M, Prommegger R, Konschake M.: Postoperative Hypoparathyroidism in Thyroid Surgery: Anatomic-Surgical Mapping of the Parathyroids and Implications for Thyroid Surgery. Sci Rep. 2019 Oct 30;9(1):15700. doi: 10.1038/s41598-019-52189-3.
- Konschake M, Zwierzina M, Moriggl B, Függer R, Mayer F, Brunner W, Schmid T, Chen DC, Fortelny R.: The inguinal region revisited: the surgical point of view : An anatomical-surgical mapping and sonographic approach regarding postoperative chronic groin pain following open hernia repair. Hernia. 2020 Aug;24(4):883-894. doi: 10.1007/s10029-019-02070-z. Epub 2019 Nov 27.
- Haid B, Pechriggl E, Nägele F, Dudas J, Webersinke G, Rammer M, Fritsch H, Oswald J.: FGF8, FGF10 and FGF receptor 2 in foreskin of children with hypospadias: an analysis of immunohistochemical expression patterns and gene transcription. J Pediatr Urol. 2020 Feb;16(1):41.e1-41.e10. doi: 10.1016/j.jpurol.2019.10.007. Epub 2019 Oct 18.
- Haid B, Reider D, Nägele F, Spinoit AF, Pechriggl E, Romani N, Fritsch H, Oswald J.: Langerhans cells in hypospadias: an analysis of Langerin (CD207) and HLA-DR on epidermal sheets and full thickness skin sections. BMC Urol. 2019 Nov 12;19(1):114. doi: 10.1186/s12894-019-0551-8.
- Czech H, Brenner E.: Nazi victims on the dissection table - The Anatomical Institute in Innsbruck. Ann Anat. 2019 Nov;226:84-95. doi: 10.1016/j.aanat.2019.03.007. Epub 2019 Apr 1.
Selection of Funding
Collaborations
- Prof. Dr. José Sanudo, MD, Prof.in Dr.in Teresa Vazquez, MD, Complutense University of Madrid, Center of Body Donation, Madrid, Spain
- Prof. Dr. Jeffrey Janis, MD, FACS, Department of Plastic Surgery, Ohio State University Medical Center in Columbus, Ohio, USA
- Prof. Dr. David Chen, MD, FACS, Lichtenstein Amid Hernia Clinic at UCLA Section of Minimally Invasive Surgery, UCLA Division of General Surgery, Los Angeles, USA
- ao Univ.Prof.Dr. Josef Oswald, FEAPU, Priv.Doz.Dr. Berhard Haid, FEBU, Department pf Pediatric Urology, Hospital of the Sisters of Charity, Ordensklinikum Linz, Linz, Austria
- ao Univ.Prof.Dr. Olaf Reich, Klinische Abteilung für Gynäkologie; Medizinische Universität Graz, Graz, Austria
- Dr. Mason Dean, Potsdam Max-Planck-Institut für Kolloid-& Grenzflächenforschung, Potsdam, Germany
- Univ.-Prof. Thomas Fichtner-Bendtsen, MD, Ass.-Prof. Jens Børglum Neimann, MD, Department of Clinical Medicine Anesthesiology, Kopenhagen, Denmark
- Univ.-Prof. Vincent Chan, MD, University Health Network - Toronto Western Hospital, Toronto, Canada
- Dr. Alexandra Butler, PhD, Larry L. Hillblom Islet Research Center, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, United States
- Prof. Dr. Juris J. Meier, St. Josef Hospital of the Ruhr-University Bochum (RUB), Bochum, Germany
 o. Univ.-Prof.in Dr.in med.univ. Helga Fritsch
o. Univ.-Prof.in Dr.in med.univ. Helga Fritsch
Director
Contact:
Müllerstrasse 59
6020 Innsbruck
Austria
Email: Helga.fritsch@i-med.ac.at
Phone: +43 512 9003 71110
Fax: +43 512 9003 73112
https://www.anatomie-innsbruck.at



