Molecular and Cellular Pharmacology
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Pharmacology, Ion Channels, Receptors, Epilepsy, Calcium Signalling
Research (ÖSTAT Classification) : 301206, 301401, 301406
Research Focus
The ‘Drexel Lab.’ investigates the contribution of GABAergic interneurons and inflammation to temporal lobe epilepsy. This laboratory combines various synergistic techniques, e.g. transgenic mice, stereotactic injections of viral vectors and monitoring of EEG activities.
The research of the ‘Di Biase Lab.’ focuses on voltage-gated calcium channels and their function with respect to synaptic transmission, plasticity and ion conduction properties. This laboratory studies the mechanisms for controlling channel trafficking and subunit assembly.
Georg Wietzorrek focuses mainly on analysing the expenditure of the Austrian health insurance system on pharmaceuticals and tries to identify potential opportunities for savings.
General Facts
The Institute for Molecular and Cellular Pharmacology is a relatively small institution in terms of associated personnel, resources and associated laboratory space. It is based on the ground floor of the Pharmacology / Genetics building at Peter-Mayr Strasse 1 and has approximately 110m2 laboratory and office space. The majority of the equipment and facilities is shared with other pharmacology units (the Institute for Biochemical Pharmacology or the Institute of Pharmacology). Our unit employs a total of 5 people. In addition to the division head and a part-time administrator, there are also 3 established, independent research groups:
Valentina Di Biase’s research group was established recently, as Valentina moved from the Medical University of Graz to Innsbruck and initiated her own research group within the Institute for Molecular Pharmacology.
Similarly, Meinrad Drexel (together with his associates) transferred from the Institute of Pharmacology and continued his epilepsy research within the Institute for Molecular and Cellular Pharmacology.
Georg Wietzorrek is longstanding member of the institute, whose focus is on clinical research with respect to public health spending and the duties of the ethics committee.
Research
Function of Calcium Channels in Neurons
Di Biase Lab
Voltage-gated calcium channels (VGCCs) are key regulators of important neuronal functions, including synaptic transmission and plasticity, gene transcription, membrane excitability, shaping of the dendritic tree and axonal guidance. Genetic studies increasingly associate calcium channel mutations with severe neurological and psychiatric diseases. However, the lack of information concerning calcium channel neurobiology is a major impediment to the assessment of channel-aetiological power and the development of novel and tailored therapeutic applications. We study the mechanisms that control calcium channel trafficking, the assembly of channel complexes and their regulation of activity, and the stimulation of G-protein-coupled receptors. We investigate how the integrity of calcium channel signalling complexes as well as their distribution and modulation affect the development of the dendritic tree, synaptic function and membrane excitability. Our experimental approach includes molecular cloning, biochemistry, electrophysiology and mainly high-resolution fluorescence imaging techniques on cultured neurons. In particular, we have developed a specialised method portfolio for cell cultures, which includes single-molecule tracking, fluorescence recovery after photobleaching, calcium imaging, synaptic fluorescent indicators and protocols of immunofluorescence – all of which are able to analyse synaptic function, signalling efficacy and channel trafficking in real time in neuronal subcellular domains.
Calcium Channels in Dendritic Complexity Regulation
Calcium channels occur upstream of competing CamKII and CamKIV pathways, respectively leading to reduction and increase in dendritic growth. Within this line of research, we address the hypothesis that channel phosphorylation is able to discriminate in the activation of signalling pathways by controlling calcium current density, channel activation and inactivation kinetics or by changing channel affinity of the interaction with molecular partners. Our results will shed light on how post-transcriptional channel regulation could act as a molecular switch between enabling or restricting dendritic tree development.
Calcium Channels in the Regulation of AMPA Receptor Synaptic Trafficking
Several lines of evidence show that calcium channels are necessary for certain forms of synaptic plasticity. The research of our laboratory shows that the pharmacological modulation of calcium channels reflects the isoform, subunit-specific, post-synaptic accumulation of AMPAR receptors. At present, we are investigating whether a putative supramolecular signalling complex, which includes calcium channel and AMPA receptors, represents the structural requirement in order to support the calcium channel-dependent synaptic trafficking of AMPA receptors.
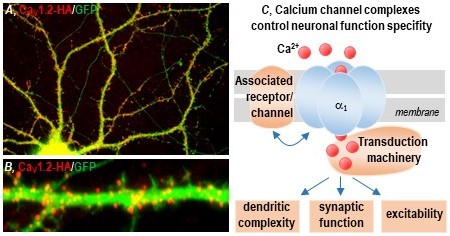
Fig. 1: Distribution of the voltage-gated calcium channel CaV1.2 isoform in the somatodendritic compartment (A) and on the dendritic shaft and spines (B) of cultured hippocampal neurons. C: Sketch summarising our research concept, analysing the impact of channel complex regulation on dendritic complexity, synaptic transmission and membrane excitability.
Drexel Lab
Research Topic 1: The Role of Hippocampal Interneurons in Epileptogenesis
In human temporal lobe epilepsy, seizures mainly arise from structures of the hippocampal formation. The electrical activity of excitatory (glutamatergic) neurons in the hippocampus is tightly controlled by a variety of distinct subclasses of GABAergic interneurons. To investigate the effect of a loss or malfunction of certain groups of GABA interneurons on epileptogenesis, we use transgenic mice, which allow cell-specific overexpression of tetanus toxin light chain (TeLC) introduced by stereotactic injections of a respective viral vector (AAV) into the hippocampus. TeLC is then selectively expressed in GABA/parvalbumin, GABA/somatostatin or GABA/VIP interneurons at the injection site and impairs neurotransmitter release from these neurons. We then monitor EEG activity in these mice for six weeks and check the development of epilepsy. To date, we have shown that selective silencing of GABA/parvalbumin neurons in the subiculum leads to spontaneous limbic seizures and a decreased seizure threshold, which highlights the crucial role of these neurons in the manifestation of temporal lobe epilepsy. More recently, we have shown that silencing of GABA/somatostatin neurons, but not GABA/VIP neurons, may also lead to the development of spontaneous recurrent seizures (i.e. epilepsy). In the future, we aim to investigate further groups of GABA interneurons in the hippocampus (containing GABA/calretinin, GABA/Chrna2 or GABA/VGLUT3).
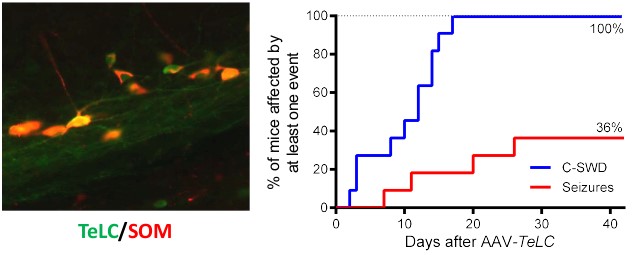
Fig. 2: Somatostatin (SOM) interneurons in hippocampal sector CA1 were functionally silenced by stereotactic injection of an AAV vector, which caused the expression of tetanus toxin light chain (TeLC). In the six weeks after vector injection, all the mice developed clusters of spike-wave discharges (C-SWD) and 36% of the mice developed spontaneous recurrent seizures.
Research Topic 2: Modulation of Inflammation in Epilepsy
Neuroinflammation is a hallmark of disease in many epilepsy syndromes and may crucially contribute to epileptogenesis and the occurrence of spontaneous seizures. Our group investigates the interplay between epilepsy, neuroinflammation and the innate immune system. Here, we focus on a key receptor family of the innate immune system: so-called toll-like receptors (TLRs). In the central nervous system, these receptors are expressed in microglia as well as – in smaller quantities – in astrocytes and neurons. While there is broad evidence that TLR4 is a crucial contributor to epileptogenesis and the occurrence of spontaneous seizures, much less is known about the other members of this receptor family. To identify a potential contribution of other TLRs to epilepsy, we are investigating some of these receptors (TLR1, TLR2, TLR5, TLR6 and TLR8) in models of acute seizures and in a model of chronic epilepsy. To date, we have shown strong upregulation of the expression of TLR2 in glial cells and neurons in several brain regions after a status epilepticus induced by intra-hippocampal injection of kainic acid. Antagonism of TLR2 signalling by a specific inhibitor (INH50) significantly attenuated a status epilepticus induced by kainate injection. In addition, by using a model of acute seizures, we showed that injection of a TLR2-specific agonist one hour before kainate injection significantly increased the number of seizures induced. These findings suggest that TLR2 signalling is a potential contributor to epilepsy and may represent a possibility for the development of novel antiepileptic medication.
Georg Wietzorrek Lab
Expenditure on pharmaceuticals is a major expense item of public health organisations. In this project, the laboratory analysed the expenditure of the Austrian national health insurance system on pharmaceuticals, as reported by the main association of social insurance institutions for the years 2006 – 2014, and calculated theoretical potential savings retrospectively.
Further research interests: pharmacology of TLR2 receptors, pharmacology of BK channels, pharmacology of hearing loss.
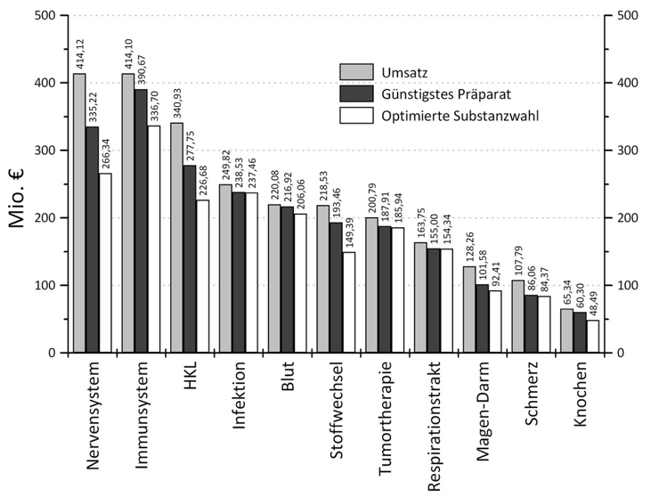
Fig. 3: Expenditure (Y axis, million EUR) on pharmaceuticals for the different indications (X axis). The grey columns represent actual expenditure of the health insurance companies; the black columns show theoretical expenditure if the cheapest drug available is chosen; the white columns show a further potential reduction in expenditure after treatment optimisation.
Selected Publications
- Bikbaev, Arthur, Ciuraszkiewicz-Wojciech, Anna, Heck, Jennifer, Klatt, Oliver, Freund, Romy, Mitlöhner, Jessica, Enrile Lacalle, Sara, Sun, Miao, Repetto, Daniele, Frischknecht, Renato, Ablinger, Cornelia, Rohlmann, Astrid, Missler, Markus, Obermair, Gerald J, Di Biase, Valentina, Heine, Martin. Auxiliary α2δ1 and α2δ3 Subunits of Calcium Channels Drive Excitatory and Inhibitory Neuronal Network Development. THE JOURNAL OF NEUROSCIENCE: 2020; 40(25): S. 4824-4841
- Drexel, Meinrad; Kirchmair, Johannes; Santos-Sierra, Sandra: INH14, a Small-Molecule Urea Derivative, Inhibits the IKK/-Dependent TLR Inflammatory Response.
2019; 20(5); 710-717. - Sperk, Günther; Kirchmair, Elke; Bakker, Jaco; Sieghart, Werner; Drexel, Meinrad; Kondova, Ivanela: Immunohistochemical distribution of 10 GABAA receptor subunits in the forebrain of the rhesus monkey Macaca mulatta. JOURNAL OF COMPARATIVE NEUROLOGY. 2020; 528(15); 2551-2568.
- Georg Wietzorrek: Analyse der Österreichischen Arzneiverordnungen 2006 – 2014; Intrinsic Activity, 2020; 8 (1), doi:10.25006/IA.8.1-k1, published online: 14 November 2020
- Hoppe K, Sartorius T, Chaiklieng S, Wietzorrek G, Ruth P, Jurkat-Rott K, Wearing S, Lehmann-Horn F and Klingler W (2020) Paxilline Prevents the Onset of Myotonic Stiffness in Pharmacologically Induced Myotonia: A Preclinical Investigation. Front. Physiol. 11:533946. doi: 10.3389/fphys.2020.533946
- Wietzorrek G, Drexel M, Trieb M, Santos-Sierra S.: Anti-inflammatory activity of small-molecule antagonists of Toll-like receptor 2 (TLR2) in mice . Immunobiology. 2019 Jan;224(1):1-9. doi: 10.1016/j.imbio.2018.11.004. Epub 2018 Nov 20.
Selection of Funding
- FWF, P33225: Dendritic complexity regulation by phospho-S1928 of CaV1.2 (Di Biase)
- FWF, P30779: Modulation of inflammation in epilepsy (Drexel)
- FWF, P31777: The role of hippocampal interneurons in epileptogenesis (Drexel)
Collaborations
DiBiase Lab:
- Martin Heine, University of Mainz, Mainz, Germany
- Johannes W. Hell, UC Davis, Davis, USA
- Matteo E. Mangoni, IGF, Montpellier, France
- Alessandra Folci, Humanitas, Milan, Italy
- Karin Hammer, University Clinic Regensburg, Germany
Drexel lab:
- Elena Pohl, University of Veterinary Medicine Vienna, Vienna, Austria
- Asla Pitkänen, University of Eastern Finland, Kuopio, Finland
- Ivanela Kondova, Biomedical Primate Research Centre, Rijswijk, The Netherlands
Devices & Services
Since the beginning of 2020 the institute is equipped with a high-density CMOS-Multielectrode array (MEA)-system. The device allows simultaneous electrophysiological recordings from 4096 electrodes arranged in a 64x64 grid and each of the electrodes may also be used for electrical stimulation. The MEA-chips are suitable for e.g., acute brain slices, organotypic hippocampal cultures, or primary neuronal cultures. We aim at establishing different in vitro models for epilepsy and seizure-like activity.
 Univ.-Prof. Dr.med.univ. Hans-Günther Knaus
Univ.-Prof. Dr.med.univ. Hans-Günther Knaus
Director
Contact:
Peter Mayer Straße 1
6020 Innsbruck
Austria
Email: Hans.G.Knaus@i-med.ac.at
Phone: +43 512 9003 70440
Fax: +43 512 9003 73440
https://www.i-med.ac.at/molpharm/



