Intensive Care and Emergency Medicine
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Devices & Services
Keywords: Acute kidney injury, sepsis, COVID-19, cardio pulmonary resuscitation, biomarkers, microvesicles, plasma pharmacokinetics, target-site pharmacokinetics, pharmacodynamics, antimicrobial agents
Research (ÖSTAT Classification) : 301103, 301205, 301203, 301401, 302013, 302030, 302031, 302032, 302053
Research Focus
- Applied clinical as well bench-to-bedside research, covering several aspects of critical illness, with special emphasis on acute kidney injury (AKI), sepsis, COVID-19 disease and cardiopulmonary resuscitation (CPR).
- Definition and clinical validation of biomarkers for diagnosis and prognosis of AKI and CPR.
- Identification and characterisation of microvesicles in severe sepsis; interaction with the coagulation system.
- Intensive-care-specific pharmacodynamics and pharmacokinetics.
General Facts
The Joint Institute / Division of Medical Intensive Care and Emergency Medicine was established in December 2012. Clinically, it was designed as a core facility for the Department of Internal Medicine, to provide a high level of intensive care and emergency medicine. It comprises a level-three intensive care unit and medical emergency room, including a short-stay (maximum of 24 hours) ward in Medizinzentrum Anichstrasse (MZA). Administratively, the unit is affiliated to the Department of Internal Medicine I (Director: Prof. Dr. Herbert Tilg).
The unit is involved in several multicentre clinical trials to investigate early diagnosis and treatment of acute kidney injury, treatment of severe infections and sepsis as well as antimicrobial pharmacokinetics. Complementary in vitro models are used to investigate inflammatory mechanisms of renal injury.
The research unit comprises the Inflammation Research laboratory (U-1-015), which hosts two research groups: the Intensive Care Medicine group (Mag. Anna Brandtner, Dr. Julia Hasslacher, Dr. Sebastian Klein, PhD, Dr. Georg Lehner, PhD, Dr. Paul Köglberger, Dr. Timo Mayerhöfer, Dr. Birgit Zassler, Mag. Viktoria Haller) led by Michael Joannidis and the Clinical Pharmacokinetics group (Tiziana Gasperetti, MSc., Jana Marx, MSc. und René Welte, PhD) led by Ao Univ. Prof. Dr. Romuald Bellmann.
Collaboration partners include all university clinics (I – V) in the Department of Internal Medicine, the Neurological Intensive Care Unit and the Surgical/Trauma Intensive Care Unit as well as the Hygiene and Medical Microbiology division and the Molecular and Cellular Pharmacology division.
Research
Intensive Care Medicine
Head: Michael Joannidis
This group is involved in the clinical research of critical illness and has a major interest in acute kidney injury and cardiopulmonary resuscitation. A second major focus is on the investigation of microvesicles in sepsis.
Acute Kidney Injury (AKI) (PI: Michael Joannidis, Sebastian Klein)
AKI occurs in critically ill patients with an incidence of > 50% and is associated with significant mortality and long-term morbidity, including end-stage renal disease. Early diagnosis and prediction of recovery are expected to have a significant impact on treatment and outcome for this syndrome. We have therefore participated in several multicentre trials to investigate biomarkers of AKI. The SAPPHIRE and OPAL study defined and evaluated urinary cell-cycle arrest proteins TIMP-2 and IGFBP-7 as early predictors of AKI with unprecedented sensitivity and specificity1. This was followed by the international multicentre RUBY study, which established the superior capability of urinary CCL14 to predict persistent AKI². In a complementary approach, we are currently investigating the effect of cell-cycle arrest on cellular recovery from damage as well as reversibility of cell-cycle arrest after relief from cell stress following a defined insult in vitro, using an endo-epithelial co-culture system. About 5 – 10% of patients who suffer from AKI require renal replacement therapy (RRT). Since optimal timing of the initiation of RRT is still insufficiently defined, we participated in the design and national coordination of what is currently the largest international multicentre randomised trial comparing accelerated versus standard initiation of RRT in critically ill patients. The results indicate that a watch and wait strategy is considered safe and avoids unnecessary RRT treatment in patients who recover quickly from AKI3. One major aetiological factor for AKI in critically ill patients is pulmonary failure; several potential pathomechanisms have been identified and are currently being investigated by the group4 in the evaluation of cross talk between injured lungs and kidneys in critically ill patients.
Hypoxic Brain Damage after Cardiopulmonary Resuscitation (CPR) (PI: Dr. Julia Hasslacher)
Cardiac arrest is one of the major causes of death in cardiovascular disease and it is frequently associated with long-term neurological deficits in case of survival. In 2014, our research group identified secretoneurin as a very sensitive and robust biomarker, which predicts unfavourable neurological outcome after CPR. In a subsequent study, we analysed additional biomarkers MRproANP and copeptin in the same cohort of 150 patients admitted to the ICU after successful CPR. Although the prediction of outcomes was acceptable, performance was clearly inferior to secretoneurin with regard to the influence of therapeutic hypothermia or haemolysis. We were recently able to confirm excellent prediction of neurological outcome after CPR by serum tau, a microtubule-associated axonal protein with a stabilising function under physiological conditions5. This predictive capability was not influenced by therapeutic hypothermia, which is a current limitation of the widely used NSE.
Severe Sepsis and Septic Shock (PI: Dr. Georg Lehner, Anna Brandtner)
Sepsis is considered to be a dysregulated host response to infection, which leads to life-threatening organ dysfunction and is estimated to account for 5.3 million deaths annually. Despite therapeutic advances and structured resuscitation protocols, mortality from this syndrome is still high, with mortality rates of up to 43.3% for patients with septic shock. A sound strategy to improve patient outcome might be to target distinct patient subgroups with specific therapeutic interventions. Thus, our research focuses on the interplay of two key players in this complex syndrome, the coagulation system and the endothelium, and we have established an in vitro coagulation test system that mimics the inflammatory reaction within the vasculature during sepsis. By using this in vitro assay, we found that different endothelium subtypes have specific procoagulatory properties: We discovered that microvascular endothelial cells exhibit the most pronounced increase in tissue factor (TF) activity upon stimulation with proinflammatory mediators, compared with endothelial cells of arterial or venous origin. Moreover, our in vitro data show that the increased TF activity in microvascular cells is the result not of higher levels of TF itself, which is a potent activator of the coagulation cascade, but rather of the imbalance of tissue factor and tissue factor pathway inhibitor (TFPI), an imbalance that is most pronounced in microvascular cells.
In current and future clinical studies, we are therefore investigating whether an increased ratio between TF/TFPI is associated with the occurrence of disseminated intravascular coagulation (DIC) and organ dysfunction in sepsis in vivo. If a high TF/TFPI ratio correlates with and predicts DIC or organ dysfunction, it might have the potential to serve as biomarker, in order to identify patients who are most likely to benefit from targeted treatment strategies that aim to modulate overwhelming procoagulatory activity caused by the TF/TFPI pathway.
COVID-19 (PI: Dr. Sebastian Klein; Timo Mayerhöfer)
Infection with SARS-COV2 carries a significant risk of respiratory failure that often requires mechanical ventilation. The surges of COVID-19 disease placed significant strain on intensive care bed capacities, leading to decompensation in some countries. At the time of the initial surge, little was known about optimal ICU resource management or major risk factors and the optimal treatment of patients. We therefore established the Tyrol COVID-19 registry, collecting information about these key issues. On the one hand, we were able to prove that an ICU network structure with a tertiary centre provided the highest level of care, as a backbone for results with superior outcome and optimal resource use. Major findings included unrecognised diabetes as the predominant risk factor for severe COVID-196 and reactivation of EBV as a common finding in the case of severe COVID-19 that is potentially associated with a more severe course of disease7.
Major achievements:
- Clinical validation of cell-cycle arrest proteins as biomarkers for AKI and urinary CCL14 for renal recovery after AKI.
- Initial identification and further evaluation of SN and serum tau as reliable early predictors of severe hypoxic brain damage after CPR.
- Establishment of an in vitro system to investigate interaction between endothelial cells and the coagulation system as well as translation of these findings into the situation of critically ill patients with sepsis.
- Epidemiology and phenotype of COVID-19 disease.
Future directions:
- Investigation of clinical interventions attenuating AKI and stimulating renal recovery in critically ill patients.
- Definition of biomarker panels for neurological outcome prediction after CPR.
Investigation of specific effects of MV subtypes on distinct proteolytic processes of the coagulation system in DIC.
Clinical Pharmacokinetics Unit
Head: Romuald Bellmann, MD, associate professor
Guided by sound pharmacokinetic and pharmacodynamic data, the optimal choice and dosage of antimicrobial agents improves clinical outcomes in critically ill patients with severe infections. The achievement of sufficient concentrations at the site of infections appears to be crucial for eradication of the causative pathogen.
The influence of continuous renal replacement therapy on pharmacokinetics of trimethoprim and sulfametrole:
The combination of trimethoprim and sulfametrole has a broad antimicrobial spectrum and is the first-line treatment for infections with Pneumocystis jirovecii and Stenotrophomonas maltophilia. We therefore assessed plasma pharmacokinetics of trimethoprim and sulfametrole in critically ill patients on and off continuous renal replacement therapy, who were treated with trimethoprim/sulfametrole. During continuous renal replacement therapy, trimethoprim and sulfametrole exposure was comparable to that of patients with approximately normal renal function. Standard doses of trimethoprim/sulfametrole therefore appear to be adequate during continuous renal replacement therapy8.
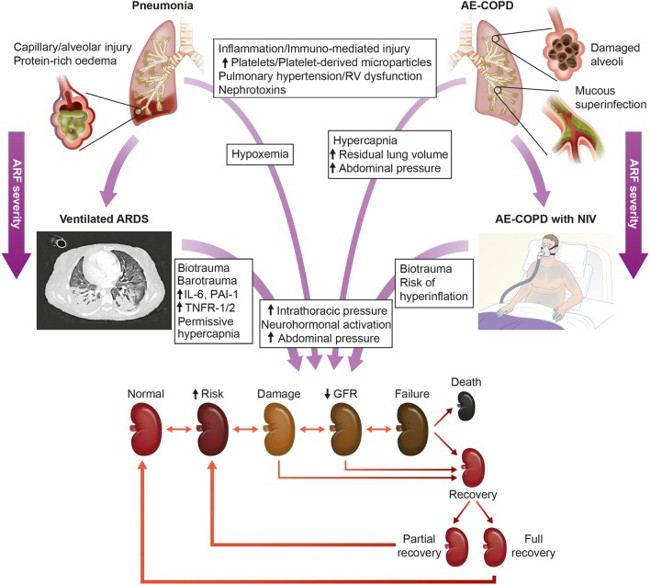
Fig. 1: Possible effects of acute respiratory failure and invasive/non-invasive ventilation on renal function. Both pneumonia and acute exacerbated COPD (AE-COPD) may trigger renal injury by various pathways. These include inflammation/immuno-mediated injury, hypoxaemia, hypercapnia and nephrotoxins. In AE-COPD, air trapping with increased thoracic pressure and right heart failure frequently contributes to venous congestion. If invasive mechanical ventilation is necessary (e.g. ARDS), then biotrauma, barotrauma, release of inflammatory mediators (e.g. IL-6, PAI-1, TNFR-1/2) and haemodynamic compromise may occur. These mechanisms may further contribute to kidney injury, eventually resulting in impaired GFR through to renal failure. Consequently, renal recovery may occur if the insulting factors are eliminated, with partial or full recovery depending on the degree of injury (www.ADQI.com).
Quantification of echinocandins in human body fluids and tissues:
Echinocandins such as anidulafungin and micafungin are recommended for the treatment of invasive Candida infections. We established and validated high-performance liquid chromatography-UV detection (HPLC-UV) assays for the quantification of anidulafungin and micafungin in human plasma, ascites fluid, pleural effusion and cerebrospinal fluid (CSF). The lower limit of quantification was 0.01 mg/l for both drugs. The methods are applicable for clinical studies on target-site pharmacokinetics of anidulafungin and micafungin8. These methods were subsequently adapted for anidulafungin and micafungin quantification in tissue samples. The lower limits of quantification were 0.05 µg/g for anidulafungin and 0.10 µg/g for micafungin9.
Target-site pharmacokinetics and pharmacodynamics of echinocandins in critically ill patients:
Data on target-site pharmacokinetics of echinocandins (e.g. in CNS) are sparse and they are largely confined to preclinical observations. We therefore quantified anidulafungin and micafungin in cerebrospinal fluid (CSF) of critically ill adults and in the cerebral cortex of deceased patients. In CSF and in the cerebral cortex, anidulafungin levels and micafungin levels were lower than in plasma. The MIC values of several pathogenic Candida strains exceed echinocandin concentrations in CSF and in the brain10.
Echinocandin target-site concentrations have recently been assessed in various body fluids of critically ill patients, i.e. in wound secretion (submitted for publication by Gasperetti et al.) and in ascites fluid where pharmacodynamics were also investigated (submitted for publication by Welte et al.) as well is in bile, pleural effusion and nine different human tissues (manuscripts in preparation).
Major achievements:
- Pharmacokinetics of trimethoprim-sulfametrole during CRRT, allowing for sound dose recommendations.
- The assessment of target-site pharmacokinetics and pharmacodynamics of echinocandins in critically ill patients revealed limited penetration of these drugs into ascites fluid, pleural effusion and wound secretion. In CSF and in brain, subtherapeutic echinocandin concentrations are to be expected.
Future goals:
- Target-site PK/PD of broad-spectrum azoles (new FWF grant).
- Completion of target-site PK/PD of echinocandins.
Selected Publications
- Joannidis M, Forni LG, Haase M, Koyner J, Shi J, Kashani K, Chawla LS, Kellum JA; Sapphire Investigators: Use of Cell Cycle Arrest Biomarkers in Conjunction With Classical Markers of Acute Kidney Injury. Crit Care Med. 2019 Oct;47(10):e820-e826.
- Hoste, Eric; Bihorac, Azra; Al-Khafaji, Ali; Ortega, Luis M.; Ostermann, Marlies; Haase, Michael; Zacharowski, Kai; Wunderink, Richard; Heung, Michael; Lissauer, Matthew; Self, Wesley H.; Koyner, Jay L.; Honore, Patrick M.; Prowle, John R.; Joannidis, Michael; Forni, Lui G.; Kampf, J. Patrick; McPherson, Paul; Kellum, John A.; Chawla, Lakhmir S.; RUBY Investigators: Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. INTENSIVE CARE MEDICINE. 2020; 46(5); 943-953.
- Bagshaw, Sean M.; Wald, Ron; Adhikari, Neill K. J.; Bellomo, Rinaldo; da Costa, Bruno R.; Dreyfuss, Didier; Gallagher, Martin P.; Gaudry, Stephane; Hoste, Eric A.; Lamontagne, Francois; Joannidis, Michael; Landoni, Giovanni; Liu, Kathleen D.; McAuley, Daniel F.; McGuinness, Shay P.; Neyra, Javier A.; Nichol, Alistair D.; Ostermann, Marlies; Palevsky, Paul M.; Pettila, Ville; Quenot, Jean-Pierre; Qiu, Haibo; Rochwerg, Bram; Schneider, Antoine G.; Smith, Orla M.; Thome, Fernando; Thorpe, Kevin E.; Vaara, Suvi; Weir, Matthew; Wang, Amanda Y.; Young, Paul; Zarbock, Alexander [Fries, D.]: Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. NEW ENGLAND JOURNAL OF MEDICINE. 2020; 383(3); 240-251.
- Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M, Prowle J, Bagshaw SM, Cantaluppi V, Darmon M, Ding X, Fuhrmann V, Hoste E, Husain-Syed F, Lubnow M, Maggiorini M, Meersch M, Murray PT, Ricci Z, Singbartl K, Staudinger T, Welte T, Ronco C, Kellum JA.: Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020 Apr;46(4):654-672.
- Hasslacher, Julia; Rass, Verena; Beer, Ronny; Ulmer, Hanno; Humpel, Christian; Schiefecker, Alois; Lehner, Georg; Bellmann, Romuald; Joannidis, Michael; Helbok, Raimund: Serum tau as a predictor for neurological outcome after cardiopulmonary resuscitation.
2020; 148(S); 207-214. - Lehner GF, Klein SJ, Zoller H, Peer A, Bellmann R, Joannidis M.: Correlation of interleukin-6 with Epstein-Barr virus levels in COVID-19. Crit Care. 2020 Nov 23;24(1):657.
- Klein SJ, Fries D, Kaser S, Mathis S, Thomé C, Joannidis M.:Unrecognized diabetes in critically ill COVID-19 patients. Crit Care. 2020 Jul 9;24(1):406
- Welte R, Beyer R, Hotter J, Broeker A, Wicha SG, Gasperetti T, Ranke P, Zaruba MM, Lorenz I, Eschertzhuber S, Ströhle M, Bellmann-Weiler R, Joannidis M, Bellmann R. Pharmacokinetics of trimethoprim/sulfametrole in critically ill patients on continuous renal replacement therapy. J Antimicrob Chemother 75:1237-1241.
- Welte R, Oberacher H, Schwärzler B, Joannidis M, Bellmann R. Quantification of anidulafungin and micafungin in human body fluids by high performance-liquid chromatography with UV-detection. J Chromatogr B Analyt Technol Biomed Life Sci 1139:121937.
- Marx J, Welte R, Gasperetti T, Moser P, Beer R, Ortler M, Jeske M, Stern R, Pomaroli A, Joannidis M, Bellmann R. Anidulafungin and Micafungin Concentrations in Cerebrospinal Fluid and in Cerebral Cortex. Antimicrob Agents Chemother 64.
Selection of Funding
Grant Support:
- COVID-19 ICU Tyrol Registry funded by the Tiroler Landesregierung
- Tissue factor and tissue factor pathway inhibitor in sepsis- ÖGIAIN Research fund
- Target-Site Pharmacokinetics and –Activity of Echinocandins, Austrian Research funds FWF (Project KLI 565-B31), Romuald Bellmann
- The study on pharmacokinetics of trimethoprim and sulfametrole was supported Austrian Research Promotion Agency (FFG, project number 848350) and by an industrial grant.
Collaborations
- Prof. Dr. Thomas Staudinger, Intensive Care Unit, Internal Medicine I, Medical University Vienna, Vienna, Austria
- Professor Stefan Kluge, Department of Intensive Care Medicine, Hamburg Eppendorf, Hamburg, Germany
- John Kellum, MD, FCCM, FACP, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
- Sean Bagshaw MD, PhD , Assoc. Prof., University of Alberta, Edmonton, Canada
- Peter Pickkers, Department of Intensive Care, Radboud University Medical Centre, Nijmegen, the Netherlands
- Ravindra L. Mehta, MD, UCSD Medical Centre, San Diego, CA, USA
- Philipp Eller, Department of Internal Medicine, University Hospital of Graz
- Daniel Dankl, Salzburg General Hospital and Paracelsus Private Medical University, Salzburg
- Piotr Smuszkiewicz, Department of Anesthesiology, Intensive Therapy and Pain Treatment, University Hospital Przybyszewskiego, Poznan, Poland
Devices & Services
- 3D Co-culture systems
- Neutrophil respiratory burst
- HPLC
- Pharmakokinetic/Pharmakodynamik consulting
 Univ.-Prof. Dr.med.univ. Michael Joannidis
Univ.-Prof. Dr.med.univ. Michael Joannidis
Director
Contact:
Anichstraße 35
6020 Innsbruck
Austria
Email: michael.joannidis@i-med.ac.at
Phone: +43 512 504 24181
Fax: +43 512 504 24199
https://www.i-med.ac.at/notfallmedizin/



